- Title
-
The role of Zic transcription factors in regulating hindbrain retinoic acid signaling
- Authors
- Drummond, D.L., Cheng, C.S., Selland, L.G., Hocking, J.C., Prichard, L.B., and Waskiewicz, A.J.
- Source
- Full text @ BMC Dev. Biol.
|
Temporal and spatial analysis of zic transcription factors and key RA synthesis and degradation genes.aldh1a2 expression is restricted to the embryonic margin at 5 hpf (A). By 8-10 hpf aldh1a2 is observed in the lateral plate mesoderm (B, C, C′). At 18 hpf, aldh1a2 is expressed in the dorsal retina and anterior somites (D). cyp26a1 is expressed in the embryonic margin and the presumptive anterior neural ectoderm at 5-8 hpf (E, F). By 10-18 hpf cyp26a1 is expressed in presumptive forebrain, midbrain, anterior hindbrain, retina, and part of the tailbud (G, G′, H). zic1 is not detectable until 8-10 hpf (75% epiboly), when it is expressed within the presumptive anterior neural tissue (I, J, K, K′). By 18 hpf, zic1 is expressed strongly in the telencephalon, midbrain-hindbrain boundary, dorsal hindbrain and spinal cord (L). zic2a initiates earlier, with dorsally-restricted expression at 5 hpf (50% epiboly) (M). By 8-10 hpf zic2a becomes anteriorly restricted within presumptive anterior neural tissue with additional midline expression (N, O, O′). By 18 hpf, zic2a is within the anterior forebrain, ventral eye, dorsal hindbrain and spinal cord (P). Initially, zic2b is expressed in a broad domain encompassing the dorsal side of the embryo (Q; 5 hpf). This broad expression is maintained at 8 hpf (R) and 10-18 hpf where expression is strongest within the eye, midbrain-hindbrain boundary, and presumptive hindbrain (S, S′, T). zic3 is also dorsally restricted at 5-8 hpf (U, V). At 10-18 hpf, zic3 is within the presumptive telencephalon, posterior forebrain, midbrain-hindbrain boundary and dorsal hindbrain, and within the tailbud (W, W′, X). Images are dorsal views with anterior to top (A-C, E-G, I-K, M-O, Q-S, U-W) or lateral views with anterior to left (C′, D, G′, H, K′, L, O′, P, S′, T, W′, X). hpf: hours post fertilization. EXPRESSION / LABELING:
|
|
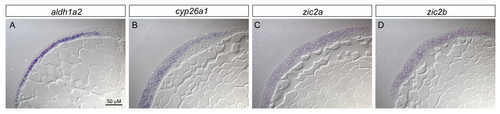
zic2a and zic2b are co-expressed with aldh1a2 and cyp26a1 in the early embryo. 90% epiboly embryos were processed for in situ hybridization for aldh1a2, cyp26a1, zic2a or zic2b, and subsequently mounted in JB-4 resin and cut into 7 μM sections on a microtome. aldh1a2 expression is clearly limited to the hypoblast (A), while cyp26a1 is present in the epiblast (B). In contrast, zic2a and zic2b each show broad expression across the germ layers (C,D). EXPRESSION / LABELING:
|
|
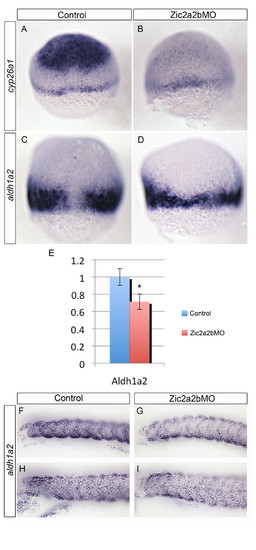
Zic2a and Zic2b depletion causes down-regulation of the retinoid-metabolism genes, cyp26a1 and aldh1a2, during early embryogenesis. mRNA in situ hybridization analysis of cyp26a1(A, B) and aldh1a2(C, D, F-I) expression reveals a reduction in both genes in Zic2a;Zic2b;p53 morpholino injected embryos (B, D, G, I) compared to control p53-morpholino injected embryos (A, C, F, H). Relative aldh1a2 mRNA expression levels for 7 hpf embryos were determined using quantitative real-time PCR (E). Levels reflect average of 7 technical replicates, with aldh1a2 mRNA at 0.71 significantly reduced (SD = 0.11; *p < 0.0001) compared to normalized control. Images are of 7 hpf embryos in dorsal view with anterior oriented to top (A-D), or lateral views with anterior to the left of 18 hpf (F, G) or 24 hpf embryos (H, I). Error bars depict standard deviation. |
|
Retinoic acid-responsive gene expression in Zic morpholino-injected embryos. mRNA transcript expression of RA-dependent markers meis3 and otx2(A, B), hnf1ba(C, D), hoxd4a(E, F), and hoxb1a(G, H) were compared in control uninjected (A, C, E, G) and Zic2a;Zic2b;Zic3MO-injected embryos (B, D, F, H). Although hoxb1a and hnf1ba are largely unchanged, a decrease in meis3 and hoxd4a is observed in Zic morpholino-injected embryos. All images are of 10.5 hpf embryos in dorsal views with anterior oriented to top. EXPRESSION / LABELING:
|
|
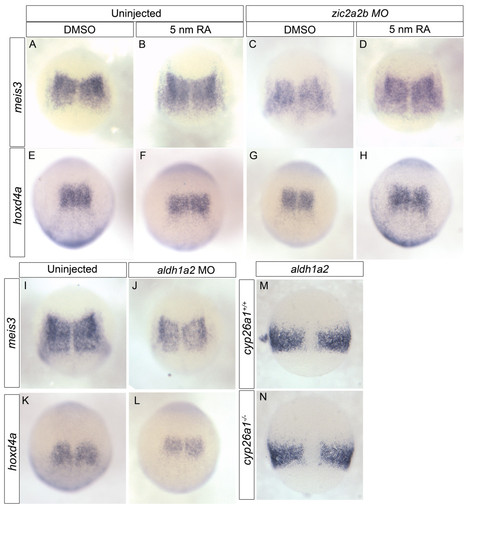
Gene expression changes upon Zic2 knockdown can be rescued by RA treatment and is mimicked by Aldh1a2 knockdown. mRNA in situ analysis of meis3(A-D, I-J), hoxd4a(E-H, K-L) and aldh1a2(M-N). Uninjected embryos were treated with DMSO (A, E) or 5 nm RA (B, F) and compared to Zic2a;Zic2bMO-injected embryos treated with DMSO (C, G) or 5 nm RA (D, H). The decrease in meis3 and hoxd4a observed in Zic morpholino-injected embryos is rescued by treatment with 5 nm RA. Aldh1a2MO-injected embryos (J, L) show a decrease in meis3 and hoxd4a when compared to controls (I, K). In cyp26a1 (giraffe) mutants (N), aldh1a2 levels remain unchanged compared to WT siblings (M). Images are dorsal views of 10.5 hpf (A-L) and 8 hpf (M-N) embryos oriented with anterior to the top. EXPRESSION / LABELING:
|
|
Hindbrain patterning in Zic morpholino-injected embryos. mRNA expression of hindbrain segmentation markers hoxa2b/aldh1a2(A, B), krox20/hoxb4a/wnt1(C, D), hoxb1a(E,F), mafba/wnt1(G, H), and egfl6(I-L) were examined in control uninjected (A, C, E, G, I, J) and Zic2a;Zic2b;Zic3MO-injected embryos (B, D, F, H, K, L). We note a reduction in hoxa2b expression in Zic morphant embryos (compare B to A) as well as a thinning of rhombomeres 3 and 5, as labeled by krox20 expression (compare D to C). Expression of hoxb4a is reduced (compare D to C), but other markers of segment identity are overtly normal (E-L). Embryos are shown in dorsal (A-H, J, L) or lateral (I, K) views and are 18 hpf. EXPRESSION / LABELING:
PHENOTYPE:
|
|
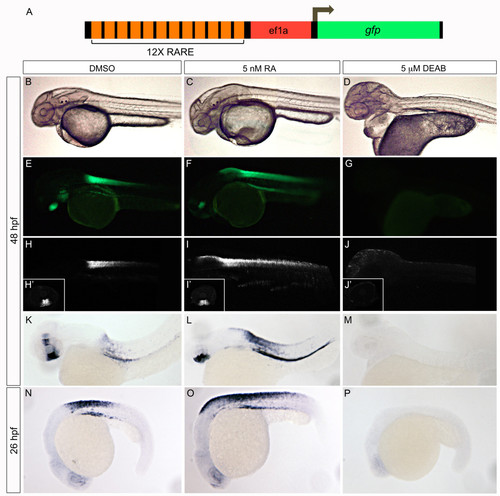
Validation of RARE:eGFP reporter. Tg(12xRARE-ef1a:eGFP)sk71 transgenic embryos (described in A) were evaluated for altered signals when RA signaling was manipulated. Embryos were treated with a vehicle control, DMSO (B, E, H, H′, K, N), 5 nM all-trans retinoic acid (C, F, I, I′, L, O), or 5 μM DEAB (D, G, J, J′, M, P). We note an expansion of hindbrain/spinal cord expression of the transgene in 5 nM RA-treated embryos, and a complete loss in 5 μM DEAB-treated embryos. Altered expression is detectable by examination of fluorescence using a stereomicroscope (B-G), confocal microscope (H-J′), or by in situ hybridization with a probe to eGFP(K-P). All embryos are shown in lateral view at 48 hpf (B-M) or 26 hpf (N-P). The inset panels (H′-J′) display reporter activity in the ventral retina. |
|
Zic2a and Zic2b depletion reduces RA signaling levels. Tg(12xRARE-ef1a:eGFP)sk71 transgenic embryos were used to assay the level of RA signaling. To increase sensitivity of transgene detection, in situ hybridization for eGFP was employed. Control p53MO-injected embryos (A, B) were compared with Zic2a;2b;p53MO-injected embryos (C, D). Quantification of reduction in eGFP in Zic2a;2b;p53 morphants is displayed graphically with reduced expression in 75 ± 2% of embryos (p-value = 0.0004; unpaired t-test). Cyp26a1 (F) and Aldh1a2 (G) morpholinos were also injected into Tg(12xRARE-ef1a:eGFP)sk71 embryos to compare effects with those obtained using Zic morpholinos. Images are of 26 hpf embryos in lateral (A, C, F, G) and dorsal (B, D) views with anterior to left and top, respectively. |
|
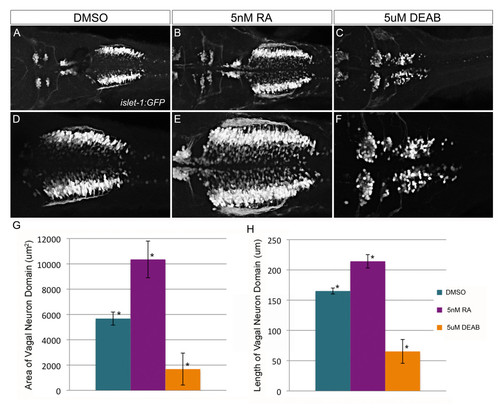
Vagal neurons are sensitive to alterations in retinoic acid levels.Tg(isl1:eGFP) transgenic zebrafish are used to visualize alterations to branchiomotor neurons in response to 1% DMSO (A, D), 5 nM all-trans retinoic acid (B, E), or 5 μM DEAB (C, F) treatment. Images are dorsal views of 48 hpf embryo hindbrain using 20× or (A-C) or 40× (D-F) objective. Length (G) and area (H) were quantified using ImageJ and displayed graphically (error bar denotes standard deviation). The differences induced by either retinoic acid or DEAB treatment are statistically significant (ANOVA and Tukey’s HSD post-hoc test, *p-value < 0.01). |
|
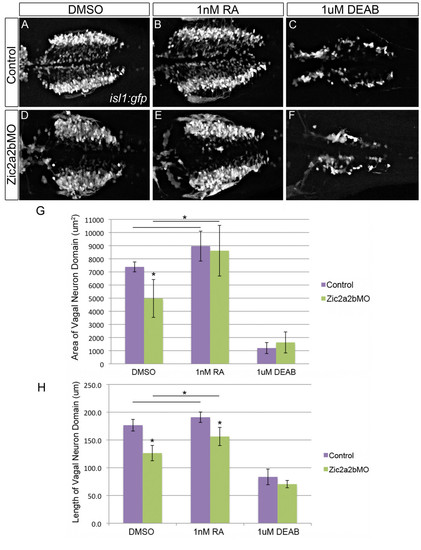
Vagal neuron domain in Zic2a and Zic2b morpholino injected embryos is partially rescued with low doses of RA. Dorsal views of the vagal motor neurons in 48 hpf Tg(isl1:eGFP) embryos that were injected with p53 morpholino (A-C) or Zic2a;Zic2b and p53 morpholinos (D-F), and treated with 1% DMSO (A, D), 1 nM RA (B, E), or 1 μM DEAB (C, F). Quantification of vagal motor neuron domain area (G) demonstrates that Zic2a/2b depletion causes a statistically significant reduction in domain size, which is rescued by treatment with retinoic acid. Quantification of vagal neuron domain length (H) gives similar results, with a statistically significant reduction upon Zic2 depletion, which could again be counteracted by retinoic acid addition. Statistical significance calculated by ANOVA and Tukey’s HSD post-hoc test (*p-value d 0.01). |
|
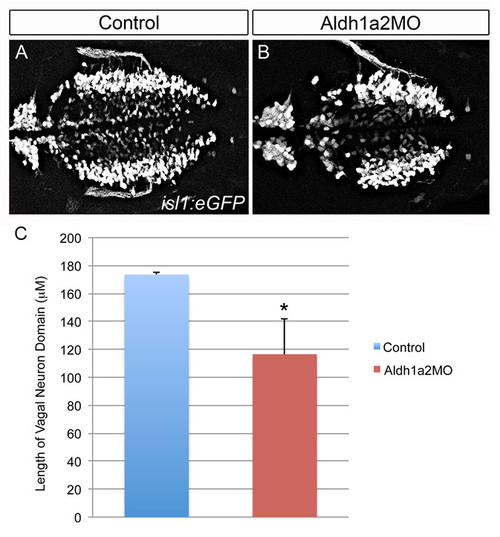
Knockdown of Aldh1a2 causes a reduction in the vagal neuron domain. Analysis of vagal motor neurons in Tg(isl1:eGFP) transgenic embryos injected with either a p53 control morpholino (1 ng, A) or an Aldh1a2 morpholino (2 ng, B). The length of the vagal domain was quantified using Image J (C). Significance calculated using an unpaired t-test (*p-value d0.01; n = 2, 6 for quantification of p53MO and Aldh1a2 MO, respectively). |