- Title
-
Low molecular weight fucoidan inhibits hepatocarcinogenesis and nonalcoholic fatty liver disease in zebrafish via ASGR/STAT3/HNF4A signaling
- Authors
- Wu, S.Y., Yang, W.Y., Cheng, C.C., Lin, K.H., Sampurna, B.P., Chan, S.M., Yuh, C.H.
- Source
- Full text @ Clin Transl Med
|
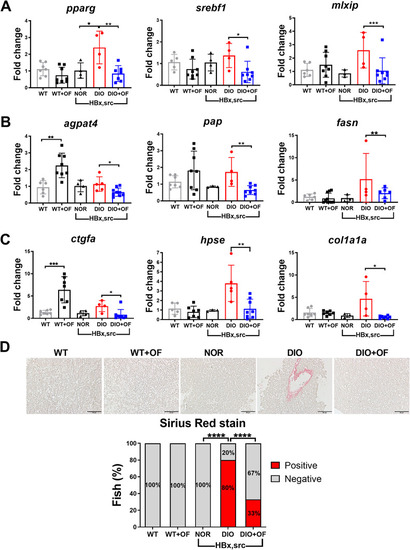
Oligo‐fucoidan pre‐treatment reduced the expression of lipogenesis genes, fibrosis marker and decreased the levels of collagen fibers in adult [ |
|
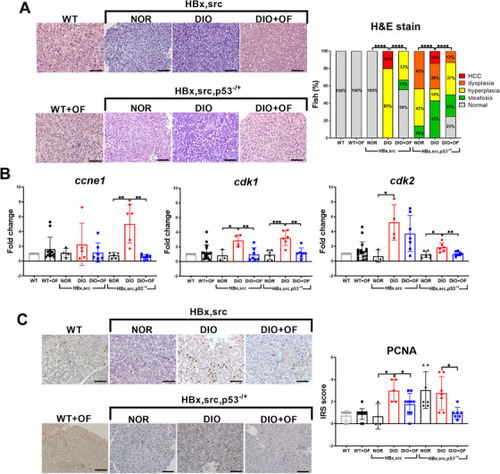
Oligo‐fucoidan pre‐treatment suppressed oncogene‐ and diet‐induced obesity‐mediated carcinogenesis in adult [ |
|
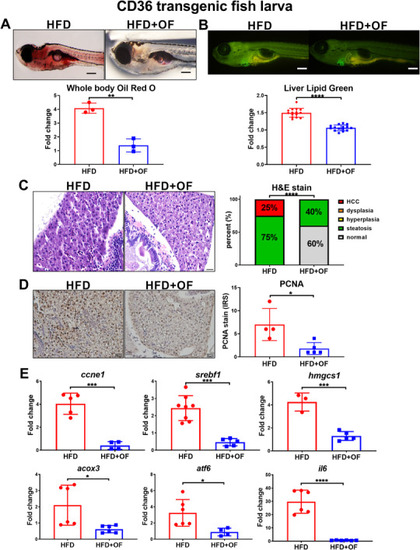
Oligo‐fucoidan exhibits anti‐lipid accumulation and anti‐HCC effect in EXPRESSION / LABELING:
PHENOTYPE:
|
|
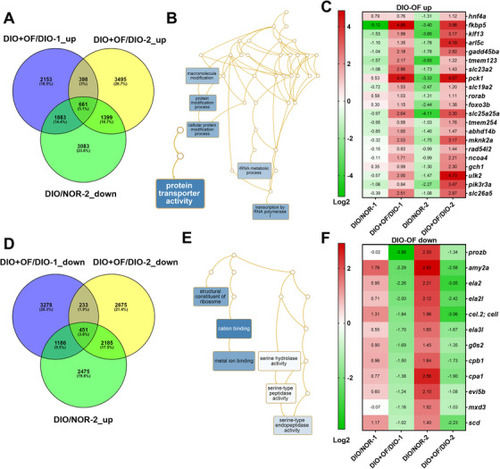
Pathways analysis for the differential expressed genes in [ |
|
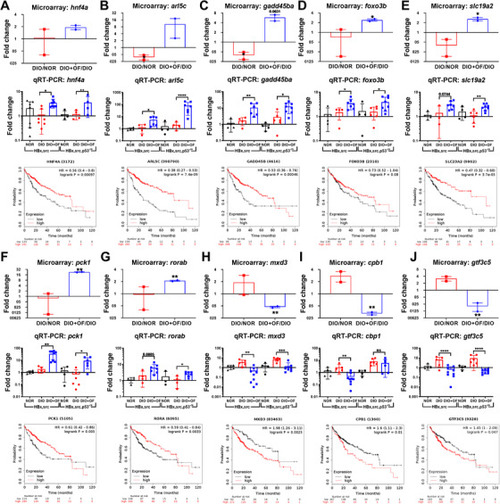
Validate the microarray data with qPCR for the five genes upregulated by OF (A‐G), and three genes downregulated by OF (H–J) in [ |
|
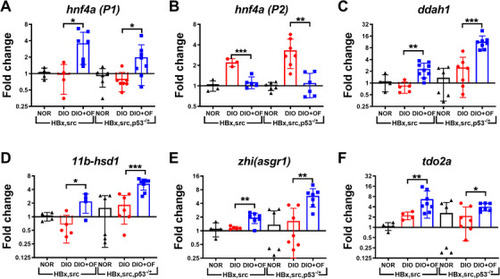
Upregulation of |
|
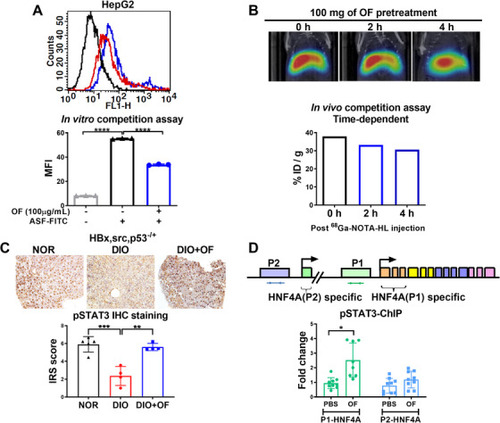
Oligo‐fucoidan binds to ASGR1/2 in hepatoma cells, enhances pSTAT3 in hepatic tissues of [ PHENOTYPE:
|
|
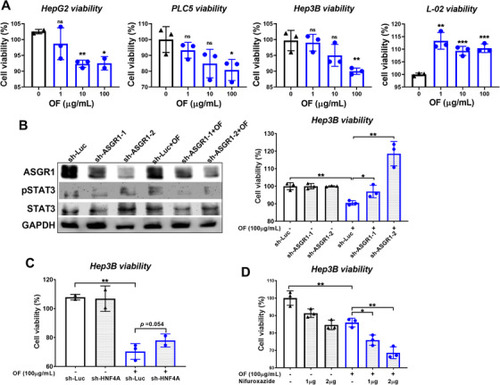
Knockdown of ASGR and HNF4A reverses the effect of oligo‐fucoidan reduced the hepatoma cell viability. A, Oligo‐fucoidan reduced the cell viability of three hepatoma cells. B, Western blot analysis indicating that knockdown of ASGR by sh‐ASGR1‐1 and sh‐ASGR1‐2 reduced the ASGR protein level and pSTAT3. Cell viability assay demonstrated that OF treated for 48 hours significantly reduced cell proliferation and sh‐ASGR1‐1 and sh‐ASGR1‐2 significantly reversed the OF effect. C, Knockdown of HNF4A by sh‐HNF4A reverse the OF inhibiting hepatoma cell viability. D, STAT3 inhibitor synergize with oligo‐fucoidan on reduction of hepatoma cell viability. |