- Title
-
Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis
- Authors
- Nica, G., Herzog, W., Sonntag, C., Nowak, M., Schwarz, H., Zapata, A.G., Hammerschmidt, M.
- Source
- Full text @ Dev. Biol.
|
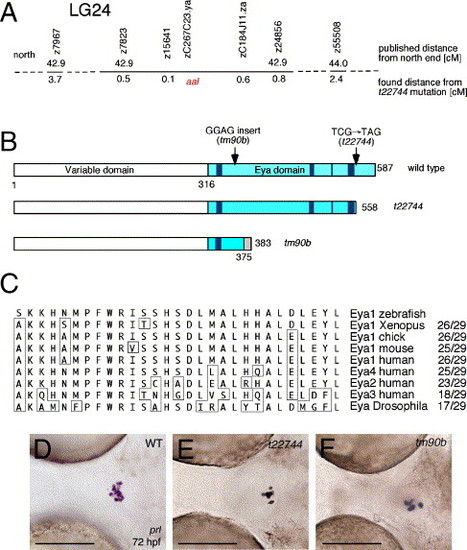
aal is a novel allele of dog encoding Eya1. (A) Genomic location of aalt22744 mutation, as determined via meiotic segregation linkage analysis. (B) Schematic drawing of wild-type and truncated Eya1 proteins encoded by the t22744 and tm90b alleles. The Eya domain is marked in light blue, the conserved noncontiguous motifs forming the catalytic phosphatase core in dark blue (aa 322-327, 492-495, 526...549-555), and the unrelated C-terminal region of tm90b downstream of the frame-shift mutation in gray. Numbers indicate amino acid residue positions. (C) Alignment of the last 29 amino acid residues of Eya1 absent in t22744. Nonconserved amino acid residues are boxed. (D–F) The pituitaries of t22744 and tm90b mutants contain similar numbers of lactotropes. prolactin in situ hybridization, ventral view on head region, anterior to the left, 72 hpf. Genotypes of embryos are shown in upper right corner (WT=wild type). Scale bars are 100 μm. EXPRESSION / LABELING:
|
|
eya1 is transiently expressed in all adenohypophyseal cells, dependent of Fgf3 and Shh function. All panels show eya1 whole-mount in situ hybridizations at stages indicated in right lower corners (h, hours post-fertilization). All embryos are oriented with anterior to the left; (A) view from animal pole, (B–I, K) lateral view, dorsal up, (J, L–P) ventral view. Arrows in panels A–D point to the anterior neural ridge, where the adenohypophyseal precursor cells are located before placode formation. Arrowheads in panels E, F point to anterior regions of the adenohypophyseal placodes, arrows to posterior lateral regions, which most likely give rise to somatotropes and thyrotropes (compare with Fig. 4A). Arrows in panels I, K, L point to the adenohypophysis, small arrowheads in panels K, L to weak eya1 staining in cells in the fenestra region of the skull, located between the pituitary and the oral cavity epithelium (compare with Fig. 8C). Panels M–P show double in situ hybridizations with eya1 in blue and pomc (M, N) or prl (O, P) in red. Panels N, P show the same embryos as in panels M, O, after the red staining had been washed out. Abbreviations: ear, inner ears; oc, oral cavity; olf, olfactory epithelium. Scale bars are 100 μm. EXPRESSION / LABELING:
|
|
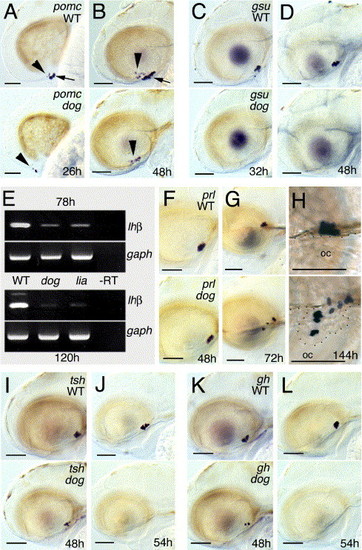
eya1 mutants fail to initiate pomc and gsuα expression and to maintain gh and tshβ expression, whereas prl expression persists. All panels except panel E show whole-mount in situ hybridizations of eya1 mutants (lower panels) and wild-type siblings (upper panels) with probes indicated in upper right corners and at stages indicated in lower right corners of lower panels; lateral views, anterior to the left. In panels A, B, arrowheads indicate pomc-positive cells of the arcuate nucleus (diencephalon), arrows indicate pomc-positive cells of the adenohypophysis. (E) Semiquantitative RT-PCR to amplify lhβ (35 cycles) or, as control, gaph (25 cycles) transcripts from pools of wild-type, or lia/fgf3 or dog/eya1 mutant larvae at 78 hpf or 120 hpf. In lia mutants, all adenohypohyseal cells die around 30 hpf (Herzog et al., 2004b), suggesting that the residual lhβ signal is due to low level hormone gene expression in tissues outside the pituitary. Similar results were obtained for fshβ and in three independent experiments. In panel H, the border of the roof of the oral cavity (oc) is outlined by dots. Note that in the eya1 mutant, parts of the adenohypophysis, including the lactotropes, have protruded into the mouth cavity. In addition, lactotropes are dispersed throughout the entire gland, rather than being confined to the anterior domain, as in wild-type siblings (compare with Herzog et al., 2003). This suggests that Eya1 function and/or Eya-dependent cell specification is required for proper adhesion between adenohypophyseal cells and/or proper segregation of the different cell types into distinct subdomains of the gland. Scale bars are 50 μm. |
|
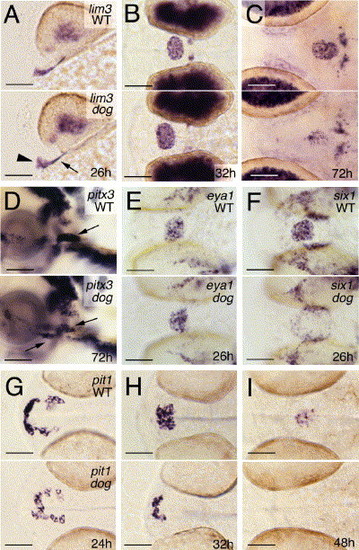
eya1 is specifically required for the transcriptional activation of pit1 and six1. All panels show whole-mount in situ hybridizations of eya1 mutants (lower panels) and wild-type siblings (upper panels) with probes indicated in upper right corners and at stages indicated in lower right corners of lower panels. (A, D) Lateral views, anterior to the left, dorsal to the top; (B, C, E-I) ventral views, anterior to the left. Arrowhead in panel A points to anterior regions of the adenohypophyseal placode, arrow to posterior lateral regions, which most likely give rise to somatotropes and thyrotropes. Arrows in panel D point to the adenohypophyseal cells, some of which are located within the oral cavity of mutant. Scale bars are 50 μm. EXPRESSION / LABELING:
|
|
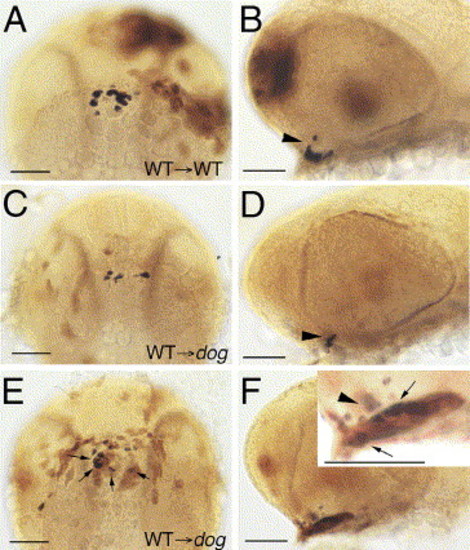
eya1 is required in adenohypophyseal cells. All panels show heads of chimeric zebrafish embryos at 32 hpf, with pomc transcripts in blue and transplanted wild-type cells in brown. (A, C, E) Ventral view, anterior to the top; (B, D, F) lateral view, anterior to the left. Inset in panel F shows magnified view of pituitary. Arrowheads in panels B, D, F point to pomc-positive cells in arcuate nucleus (ventral diencephalon) (Herzog et al., 2003), arrows in panels E, F to pomc-positive cells in adenohypophysis. Note that many, but not all of the transplanted wild-type cells in the adenohypophysis of the eya1 mutant in panels E, F display pomc expression. pomc-negative wild-type cells most likely represent other cell types (lactotropes, somatotropes, thyrotropes, and/or gonadotropes). Scale bars are 50 μm. |
|
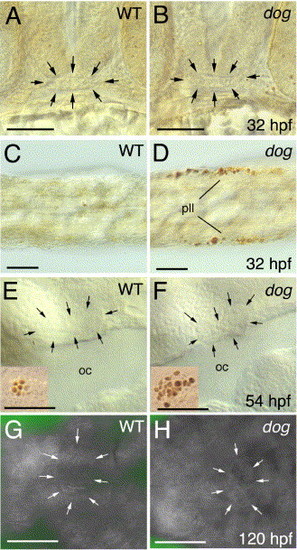
The adenohypophysis of eya1 mutants shows normal apoptosis rates. Panels A-F show TUNEL stainings, panels G, H acridine orange stainings of eya1 mutants and wild-type siblings at stages indicated in lower right corners (hpf; hours post-fertilization). (A, B) Frontal views on heads, dorsal up. (C, D) Dorsal views on tails of same embryos shown in panels A, B, showing apoptosis of cells in the posterior lateral line primordium (pll). (E, F) Lateral views on heads at level of oral cavity (oc). (G, H) Ventral views on heads. Adenohypophysis in panels A, B, E-H is outlined by arrows. Insets in panels E, F show, as internal control, apoptotic cells from inner ear region of same specimen (with more apoptotic cells in mutant; compare with Kozlowski et al., 2005). Scale bars are 50 μm. |
|
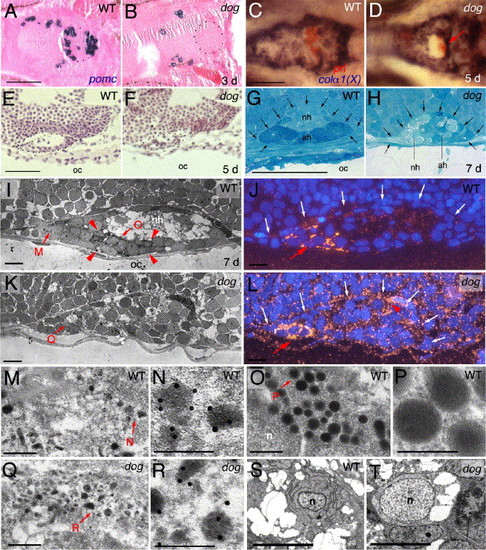
The adenohypophysis of eya1 mutants is of moderately reduced size, with the majority of cells lacking morphological features characteristic for secretion processes. (A, B) Sagittal section after in situ hybridization for pomc, counterstained with eosin/hematoxylin; 3 days post-fertilization (d), anterior to the left. Borders of the pituitary are outlined with dots. (C, D) Double in situ hybridization for prolactin in red and collagen alpha 1(X) in blue, 5 days, ventral view, anterior to the left. prl-positive cells in panel D are indicated with red arrow. (E, F) Longitudinal section stained with eosin/hematoxylene, 5 days, anterior to the left, dorsal to the top. (G, H) Longitudinal section stained with toluidine blue, 7 days, anterior to the left, dorsal to the top. The borders of the pituitary, now with a distinct adenohypophysis (ah) and a neurohypophysis (nh), are indicated by arrows. Note the severe reduction of tissue separating the pituitary from the oral cavity in the mutant (H). (I, K, M–R) Anti-prolactin immunogold electron micrographs of longitudinal sections, anterior to the left, dorsal to the top, 7 days. (I, K) Overviews over entire pituitaries. Red arrowheads in panel I demarcate region with eight type 2 cells in the wild-type pituitary, which are absent in the mutant (K). Panels J, L show adjacent sections of the same specimen as in panels I, K after fluorescent detection of Prl and DAPI staining of nuclei; dorsal borders of the pituitaries are indicated by white arrows. Red arrows in panels J, L point to rostral region with six Prl-positive cells in wild-type (J), three in mutant (L); red arrowhead in panel L indicates region with Prl-positive cells in more lateral sections of the same mutant pituitary, consistent with the randomized and ectopic positions of prl-positive cells in mutant pituitaries revealed via in situ hybridization (compare with Fig. 3H). Panels M, O, Q show higher magnifications of regions indicated in panel I or K, with type 1 cells in panels M, Q, and a type 2 cell in panel O; panels N, P, R show higher magnifications of regions indicated in panels M, O, or Q, revealing electron-dense gold particles in panels N and R, but not in panel P. (S, T) Electron micrographs with ultrastructure of wild-type (S) or mutant (T) adenohypophyseal cell lacking secretory granules. Both cells contain vacuole-like structures, while only the wild-type cell has an elaborated endoplasmatic reticulum. Abbreviations: ad, adenohypophysis; nh, neurohypophysis; n, nucleus; oc, oral cavity. Scale bars are 50 μm for panels A–H, 5 μm for panels I–J, S, T, 0.5 μm for panels M, O, Q, and 0.25 μm for panels N, P, Q. EXPRESSION / LABELING:
|
|
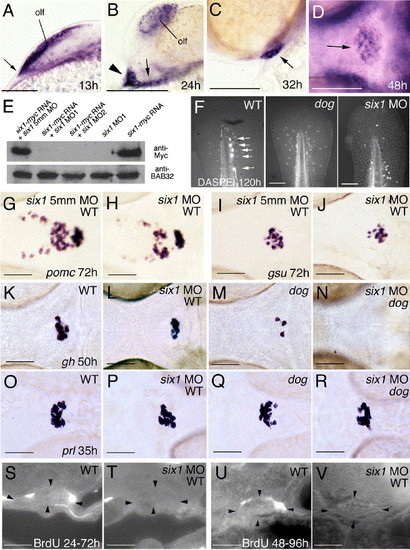
Knockdown of six1 has no effects on lineage-specific differentiation steps in wild-type embryos, while affecting cell proliferation and moderately enhancing the specification defects of eya1 mutants. (A–D) six1 is expressed similarly to eya1. Panels show six1 in situ hybridizations at stages indicated in lower right corners. (A–C) Lateral views on head, anterior to the left, dorsal to the top; (D) ventral view, anterior to the left. Arrow in panel A points to anterior neural ridge (compare with Fig. 2C), arrowhead and arrow in panel B to anterior and posterior lateral domains of the adenohypophyseal anlage, respectively (compare with Fig. 2E), and arrows in (C, D) to adenohypophysis. (E) six1 morpholino oligonucleotides (MO) efficiently block synthesis of Six1-Myc protein encoded by co-injected mRNA, whereas the 5 mismatch (5mm) control MO does not. Anti-Myc and (as control) anti-BAB32 Western blot analysis of protein extracts from injected embryos at 10 hpf. Injected MOs and/or mRNAs are indicated. (F) DASPEI staining of posterior lateral line neuromasts in the tip of the tail of wild-type larva at 120 h (left panel; indicated by arrows), which is absent in dogt22744 mutant (middle panel) and six1 morphant (right panel); posterior up, dorsal to the left. (G–L, O, P) Wild-type embryos injected with six1 MO (H, J, L, P) display expression of pomc, gsuα, gh, and prl, however, compared to siblings injected with the 5mm control MO (G, I) or uninjected siblings (K, O), numbers of all cell types are moderately reduced. (K–N) eya1 mutant injected with six1 MO (N) lacks gh expression at stages when uninjected eya1 mutant sibling (M) still contains a few gh-positive cells, while the number of gh-positive cells in six1 MO-injected wild type embryos (L) is only moderately reduced compared to un-injected control (K). (O–R) eya1 mutant injected with six1 MO (R) contains several prl-positive cells, comparable to the number of prl-positive cells in six1 MO-injected wild-type embryo (P). (S–V) In wild-type animals, a few adenohypophyseal cells have incorporated BrdU between 24 hpf and 72 hpf (S) or between 48 hpf and 96 hpf (U), whereas no BrdU-positive cells were detected in six1 morphants (T, V). Abbreviations: olf, olfactory epithelium. Scale bars are 100 μm for panels A–D, F, 50 μm for panels G–V. EXPRESSION / LABELING:
|
|
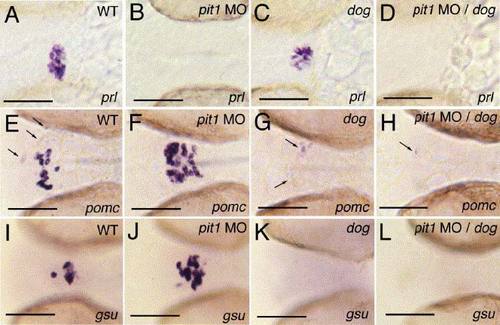
pit1 morphant eya1 mutants lack expression of all hormone genes. All panels show ventral views on the head region of embryos at 32 hpf, after in situ hybridization with probes indicated in lower right corners. (A, E, I) Wild type; (B, F, J) wild type injected with pit1 antisense morpholino oligonucleotide (pit1 MO); (C, G, K) eya1 mutant; (D, H, L) eya1 mutant injected with pit1 MO. Arrows in panels (E, G, H) point to pomc-positive cells in the arcuate nucleus. Scale bars are 50 μm. EXPRESSION / LABELING:
|
|
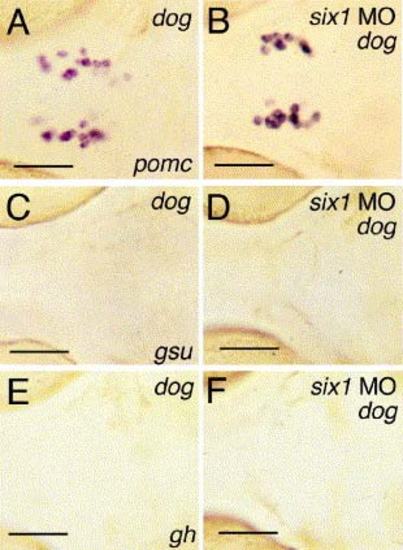
Loss of Six1 activity in eya1 mutants does not rescue adenohypophyseal hormone gene expression. All embryos are at 72 hpf, ventral views, anterior to the left, after in situ hybridization with probes indicated in right lower corners of panels (A, C, E). (A, C, E) Uninjected dog controls; (B, D, F) dog mutants injected with six1 MO1. pomc staining in panels (A, B) is confined to arcuate nucleus cells. Scale bars are 50 μm. EXPRESSION / LABELING:
|

Unillustrated author statements |
Reprinted from Developmental Biology, 292(1), Nica, G., Herzog, W., Sonntag, C., Nowak, M., Schwarz, H., Zapata, A.G., Hammerschmidt, M., Eya1 is required for lineage-specific differentiation, but not for cell survival in the zebrafish adenohypophysis, 189-204, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.