- Title
-
Notch regulates blastema proliferation and prevents differentiation during adult zebrafish fin regeneration
- Authors
- Münch, J., González-Rajal, A., and de la Pompa, J.L.
- Source
- Full text @ Development
|
Notch signalling is activated during fin regeneration. (A) qPCR analysis of notch1b, lfng, her6 and her15 mRNA in regenerating fins at 3 dpa relative to expression in non-amputated fins (0 dpa). *P<0.05, **P<0.01. (B-F) Whole-mount in situ hybridization in 2 dpa fins for jag1b (B), notch1b (C), lfng (D), her6 (E) and her15 (F), showing Notch pathway activation within the blastema (arrowheads). (G) Scheme of a 3 dpa fin section, indicating the two regions within the regenerate: the blastema (b) and the differentiation zone (dz). Green indicates regenerating fin rays. (H-J) In situ hybridization on 3 dpa fin sections for jag1b (H), lfng (I) and her6 (J) reveals Notch pathway activation in the blastema (b, arrowhead), but weak or no gene expression within the dz (arrow). Scale bars: 100 μm in C,F; 10 μm in J. Broken lines mark amputation plane. EXPRESSION / LABELING:
|
|
Lunatic fringe-mediated Notch signalling in proliferating blastema cells. (A-B2) EGFP expression in the enhancer trap line ET33mi60 is weak in non-amputated fins (A,A2), but strong in the fin regenerate at 3 dpa (B,B2). (C-E2) Time-course of ET-33mi60A regenerating fins. No EGFP expression is seen in the wound epidermis at 1 dpa. (C,C2). EGFP expression starts at 2 dpa within the blastema (D,D2, arrows). EGFP expression is strong within the blastema at 3 dpa (E,E2, arrows). (F,F2) her6 in situ hybridization and EGFP immunohistochemistry on a fin at 3 dpa reveals EGFP expression in cells in which Notch is activated (arrows). (G-I) EGFP and PCNA double immunohistochemistry. (G,H) Confocal microscopy images: EGFP is expressed during blastema formation (2 dpa) in cells co-expressing PCNA (G, arrowheads). At 3 dpa, EGFP expression is strong in all blastema cells (H, arrowheads), and is mosaic in proximal regions (H, arrows). Most cells are co-labelled with PCNA (H,I, arrowheads). At 5 dpa, EGFP expression is strong in the blastema (I, arrowheads) but weak in the proximal region (I, arrows). Most cells in the EGFP+ area express PCNA (I, arrowheads). Scale bars: 1 mm in B2 100 μm in E; 10 μm in E2,I. Broken lines mark the amputation plane. EXPRESSION / LABELING:
|
|
Notch signalling inactivation impairs regeneration by decreasing proliferation. (A,B) Fish were treated with DMSO or 10 µM RO4929097 for 3 days starting at 1 hpa. Fin regeneration is blocked by RO4929097 treatment. (C) Mean length of fin regenerates (n=5); **P<0.01. (D,E) In situ hybridization of her6 showing Notch activity in regenerating fins treated with DMSO from 62 to 72 hpa (D) but not in RO4929097-treated fins (E). (F-H) BrdU-stained fin sections and quantification of BrdU+ cells within the mesenchyme of RO4929097-treated fins (G; n=5) and DMSO-treated fins (F; n=6). *P<0.05. (I-M) Fins microinjected in the dorsal half with fluorescein-labelled morpholinos (MO, green) and electroporated at 2 days post-transfection (dpt): control (I; n=9), jag1b (J; n=12), notch1b (K; n=9), lfng (L; n=12) or rbpjκ (M; n=6). The ventral half serves as an internal control. Pink brackets mark maximal regenerative outgrowth at 2 dpt. (N) Mean outgrowth size of the dorsal (MO-electroporated) half of the fin relative to the ventral half at 4 dpa/2 dpt. *P<0.05, **P<0.01 and ***P<0.005 versus control. (O,P) BrdU-stained fin sections and quantification of BrdU+ cells within the mesenchyme. (Q) Mean percentage of DAPI+ blastema cells in fin sections incorporating BrdU at 3 dpa/24 hpt (n=3). *P<0.05. Proliferation is reduced in notch1b-MO-transfected fin halves. Scale bars: 1 mm in B; 10 μm in E,G; 1 mm in M; 10 μm in P. Broken lines indicate the amputation plane. |
|
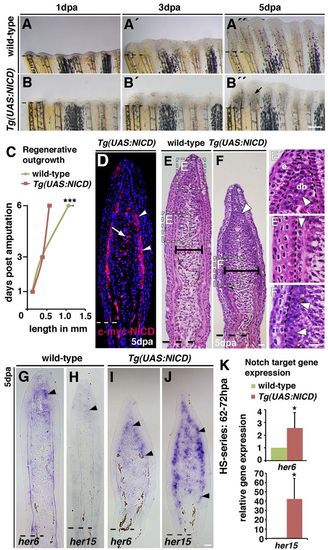
Notch gain of function leads to blastema expansion and impaired fin regeneration. Wild-type and Tg(hsp70l:Gal4);Tg(UAS:myc-notch1a-intra) fish, referred to as Tg(UAS:NICD), were heat-shocked throughout the regeneration period. (A-B3) Live images of wild-type and Tg(UAS:NICD) fins at 1, 3 and 5 dpa. No phenotypic differences were observed at 1 (A,B) and 3 (A2,B2) dpa between wild-type and Tg(UAS:NICD). Regenerative outgrowth progresses in 5 dpa wild-type fish (A3) but is blocked in Tg(UAS:NICD) fish (B3). The blastema is swollen proximal to each fin radial (B3, arrow). (C) Mean length of the regenerate at 1, 3 and 6 dpa: regenerative outgrowth is significantly reduced in Tg(UAS:NICD) fish at 6 dpa; ***P<0.001. (D) Immunohistochemistry against the Myc tag on the NICD transgene (Myc-NICD) in 5 dpa Tg(UAS:NICD) fin sections. Expression of Myc-NICD is mosaic in the blastema, in two or three cell layers beneath the epidermis (arrowheads) and in intraray blastema cells (arrow). (E-F2) Haematoxylin and Eosin stained fin sections (5 dpa): the blastema of regenerated Tg(UAS:NICD) fins is broader than wild-type blastema (E,F, horizontal bars; supplementary material Fig. S4C). Higher magnification views of wild-type fins reveal densely packed distal blastema cells (E3, arrowhead), the looser organization in the central proximal region and the strict alignment in the periphery (E3, arrowhead). In Tg(UAS:NICD) fins, cells are densely packed and disorganized in both blastema regions (arrowheads, F,F2). This structure is similar to the blastema (E2,F,F2). (G-J) In situ hybridization at 5 dpa. her6 and her15 transcription expands into the proximal blastema region in Tg(UAS:NICD) fins (arrowheads, G-J). (K) qPCR analysis after a 10-hour heat-shock series (62-72 hpa). *P<0.05. Scale bars: 200 μm in B310 μm in F,F2,J. |
|
Notch gain of function increases proliferation and is accompanied by an expansion of distal blastema marker expression into proximal regions. (A-C) BrdU-stained fin sections after a 10-hour heat-shock series (62-72 hpa) and quantification of the percentage of BrdU-labelled DAPI nuclei in the blastema: more cells incorporate BrdU in Tg(UAS:NICD) fins (B) than in wild-type fins (A). Asterisk in B indicates autofluorescent blood vessels. *P<0.05. (D-I) msxb, msxe and aldh1a2 in situ hybridization (5 dpa). Arrowheads indicate expression of msxb, msxe and aldh1a2. (J) qPCR analysis after a 10-hour heat-shock series (62-72 hpa): msxb, msxe and aldh1a2 transcripts are increased in Tg(UAS:NICD) fins. *P<0.05. Scale bars: 10 μm in B,I. |
|
Notch signalling inhibits osteoblast differentiation and prevents bone regeneration. (A,B) Alizarin Red staining of fins at 5 dpa reveals calcified bony rays in a wild-type fin regenerate (A, arrow). Fin rays are poorly formed in Tg(UAS:NICD) fins, whereas the blastema (b) is expanded (B, arrow). (C) Length of regenerated calcified radials of Tg(UAS:NICD) fins, relative to wild-type fins. ***P<0.001. (D,E) In situ hybridization at 5 dpa. Wild-type fins express tcf7 in peripheral cells in the distal region (D, arrow), whereas transcription is expanded proximally in Tg(UAS:NICD) fins (E, arrow). (F,G) Immunostaining for Osx and Myc-NICD. In wild-type fins, Osx+ cells (green) are present in the proximal fin region but scarce in the distal region (F, bracket). Osx+ cells are less numerous in regenerating Tg(UAS:NICD) fins and more proximally limited. The region of high Myc-NICD expression does not contain Osx+ cells (G, bracket). (H) Relative length of the proximal region of the fin regenerate, which contains Osx+ cells, and the distal region (brackets, F,G), which does not (Osx-). The distal, Osx- region is larger in Tg(UAS:NICD) fins than in wild-type fins. *P<0.05. Scale bars: 200 μm in B; 10 μm in E,G. Broken lines indicate the amputation plane. |
|
Notch signalling is active in the blastema. (A) qPCR determination of deltaC, deltaD, jag1b and notch1a transcripts in regenerating fins at 3 dpa relative to non-amputated fins (0 dpa). (B,C,E) In situ hybridization on 3 dpa fin sections. msxb is expressed in the whole blastema (B) whereas msxe (C) and aldh1a2 (E) expression is restricted to the distal region (arrowheads) but absent in the proximal region (arrows). (D) BrdU-labelled cells are densely packed in the distal region of the blastema (arrowhead), but dispersed proximally (arrow). (F-K) In situ hybridization on 5 dpa fin sections. jag1b (F), lfng (G) and her6 (H) are expressed in the distal region of the blastema (arrowheads) but not in the more proximal differentiation zone, similar to msxe (I) and aldh1a2 (K). Scale bar: 10 μm in E,K. Broken lines mark the amputation plane. |
|
Lunatic-fringe-mediated Notch signalling is activated in proliferating, aldh1a2-expressing cells. (A) Mean percentage of EGFP-expressing cells in the regenerate of ET33-mi60A fin sections at 2 dpa, 3 dpa and 5 dpa. ***P<0.05. (B) Mean percentage of EGFP+ blastema cells co-labelled for PCNA in fin sections at 2 dpa, 3 dpa and 5 dpa. (C) Representative immunhistochemistry for EGFP and aldh1a2 in a 3 dpa fin section. Cells are double positive in the distal region of the blastema (arrowhead). Scale bars: 100 μm in C. Broken line marks the amputation plane. |
|
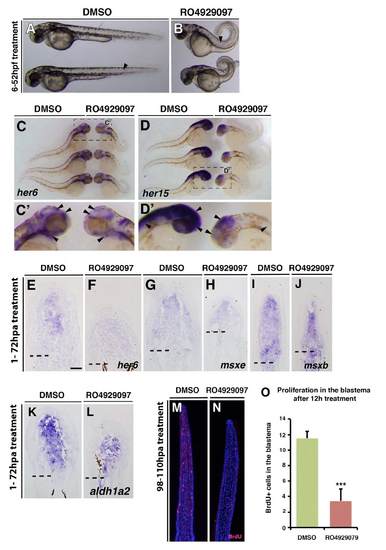
RO929097 treatment leads to Notch signalling knockdown in embryos and regenerating fins and reduces proliferation. (A-D) Embryos treated with either DMSO or 10 μM RO929097 from 6 to 52 hpf. RO929097-treated embryos show defects in somitogenesis (arrow) and a looped tail (B). (C,D) Whole-mount in situ hybridization: her6 gene expression is reduced in the brain and the gill mesenchyme of RO929097-treated embryos (arrowheads). her15 gene expression is reduced in the brain and spinal cord of RO929097-treated embryos (arrowheads). (E-L) In situ on fin sections after 72 hours of DMSO or 10 μM RO929097 treatment. her6 gene expression is reduced in RO929097-treated fins (E,F), but msxe (H), msxb (J) and aldh1a2 (L) expression seems to be unchanged in RO929097-treated compared with DMSO-treated fins (G,I,K). (M-O) Anti-BrdU-stained fin sections and quantification of BrdU+ cells within the distal most 300 μm of the mesenchyme. RO4929097-treated fins (n=4) (N) exhibit fewer BrdU-labelled cells than DMSO-treated fins (M) (n=4). ***P<0.001. |
|
DAPT treatment leads to Notch signalling downregulation in regenerating fins and reduces regenerative outgrowth. (A,B) Juvenile fish treated with 50 μM DMSO or DAPT for 3 days. Fin regeneration is impaired by DAPT treatment. (C) Mean length of fin regenerates; DAPT treatment (n=10) decreased regenerate length compared with DMSO-treated fins (n=6); ***P<0.005. (D,E) her6 whole-mount in situ hybridization reveals Notch activity in DMSO-treated fins (D) but not in DAPT-treated fins (E). |
|
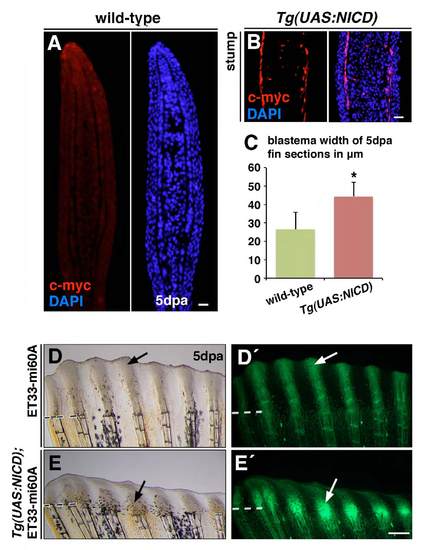
NICD overexpression leads to increased blastema width and increases EGFP expression in Tg(UAS:NICD);Et33-mi60A fish. (A,B) Immunohistochemistry for Myc does not label wild-type fin sections (A), whereas Myc-NICD expression is detected in peripheral cells, most likely osteoblasts, and cells within the ray in the fin stump of Tg(UAS:NICD) fish (B). (C) Mean blastema width of wild-type and Tg(UAS:NICD) fin sections at 5 dpa. ***P<0.05. (D-E′) Tg(UAS:NICD) fish were crossed with Et33-mi60A fish and the Tg(UAS:NICD);Tg Et33-mi60A fish were exposed to a series of heat shocks during 5 days of regeneration. EGFP expression is increased in Tg(UAS:NICD);ET33-mi60A fish at 5 dpa (E9) compared with ET33-mi60A fish. Scale bars: 10 μm. Broken lines mark the amputation plane. |
|
Notch signalling pathway overactivation leads to increased blastema marker expression and higher proliferation. (A-H) In situ hybridization of fins after a 12-hour heat-shock period (98-110 hpa). her6, her15, msxb and msxe expression is present in the distal region of the blastema in wild-type fins (A,C,E,G). Gene expression is stronger and expanded proximally in Tg(UAS:NICD) fish (B,D,F,H). (I) Double immunohistochemistry reveals co-labeling of Myc-NICD and BrdU in many cells (arrowheads). (J-K′) Immunohistochemistry of PCNA and aldh1a2 on 5 dpa fin sections. Double labelled cells (J9,K9) are restricted to the distal region of the wild-type fin (J, arrowhead) but is expanded proximally in Tg(UAS:NICD) fins at 5 dpa (K, arrowheads). (L,M) Immunohistochemistry against Osx of fins after a 12-hour heat-shock period (98-110 hpa). Scale bars: 10 μm in H,I,K,M. Dashed lines indicate the amputation plane. |
|
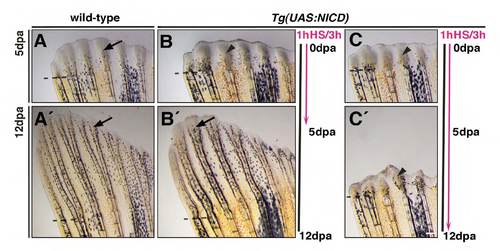
NICD-induced blastema expansion is reversible. (A-C′) Heat-shock cycles were applied over 5 days of regeneration (A-B′) or 12 days (C,C′). Black line indicates time of regeneration. Pink line indicates time of heat-shock treatment (1 hour of heatshock every 3 hours for 5 or 12 days). Arrows indicate regenerating radials. Arrowheads indicate expanded blastemas. Regeneration is inhibited in Tg(UAS:NICD) (B,C) fins but not in wild-type fins (A). Regeneration reverts to normal when heat-shock treatment is stopped (A′,B′, arrows) but the blastema remains close to the amputation plane (arrowhead) when heat-shocked continued up to 12 days (C′). Scale bars: 100 μm. Dashed lines mark the amputation plane. |