- Title
-
Identification of early requirements for preplacodal ectoderm and sensory organ development
- Authors
- Kwon, H.J., Bhat, N., Sweet, E.M., Cornell, R.A., and Riley, B.B.
- Source
- Full text @ PLoS Genet.
|
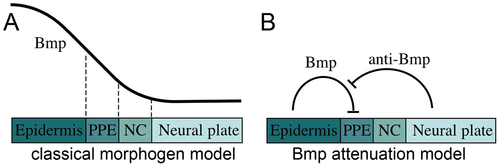
(A) Classical model in which a Bmp morphogen gradient directly specifies multiple fates, including epidermal ectoderm, preplacodal ectoderm (PPE), neural crest (NC) and neural plate, at discrete threshold concentrations. (B) Bmp-attenuation model in which Bmp-antagonists, secreted from the dorsal tissue of the embryo, promotes preplacodal fate in nonneural ectoderm abutting the anterior neural plate. In this model, Bmp must be fully blocked to permit preplacodal specification. |
|
Distinct responses of neural crest and preplacodal ectoderm to graded impairment of Bmp. (A) Lateral views of foxd3 expression at 11 hpf with anterior up and dorsal to the right. Embryos were treated with indicated concentrations of DM added at 4 hpf. Where indicated the DM concentration was increased to 200 µM (complete Bmp-inhibition) at 5 hpf, 6 hpf or 7 hpf. (B) Lateral views of six4.1 expression at 11 hpf in embryos treated with indicated concentrations of DM beginning at 4 hpf. Treatment with 25 µM DM yields two discrete responses, one in which six4.1 remains confined to two bilateral stripes flanking the neural plate and the other in which six4.1 expression is lost. (C) Lateral views showing expression of preplacodal competence factors tfap2a, tfap2c, foxi1 and gata3 in embryos were treated with 50 µM DM beginning at 4 hpf. Note that tfap2a/c remain broadly expressed in ventral ectoderm whereas foxi1 and gata3 are nearly eliminated. EXPRESSION / LABELING:
PHENOTYPE:
|
|
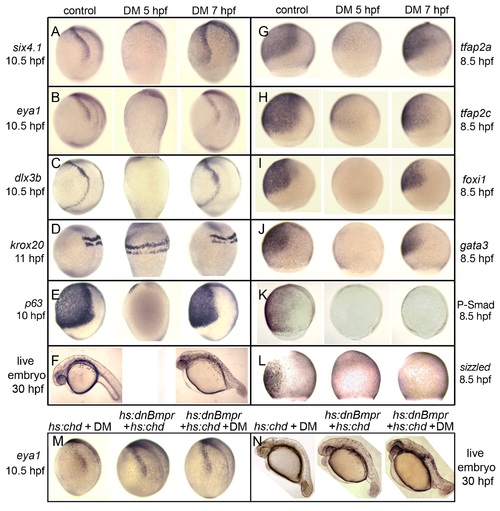
Stage-dependent requirements for Bmp. (A–E, G–L) Analysis of indicated gene expression patterns in control embryos and embryos treated with 100 µM dorsomorphin (DM) at 5 hpf or 7 hpf. Lateral views with dorsal to the right and anterior up. Expression of six4.1, eya1 and dlx3b (A–C) in PPE, krox20 in hindbrain(D) and p63 in epidermal ectoderm (E). Expression of competence factor genes tfap2a, tfap2c, foxi1 and gata3 (G–J). Reporters of Bmp-signaling, Phospho-Smad1/5/8 antibody staining (K) and sizzled in situ hybridization (L). Note the complete loss of Bmp signaling by 100 µM DM-treatment either at 5 hpf or 7 hpf. (F) Lateral views of live embryos at 30 hpf. Embryos treated with DM at 7 hpf show a partially dorsalized C3 phenotype [33]. (M, N) Tg(hs:chd) and/or Tg(hs:dnBmpr) embryos heat-shocked and treated with 100 µM DM at 7.5 hpf. eya1 expression (M) and C3 phenotypes (N) are comparable to embryos treated with 100 µM DM alone. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Formation of cranial placodes requires competence factors but not Bmp during gastrulation. Analysis of various cranial placode markers in control embryos, embryos treated with 100 µM DM at 7 hpf, or foxi1/gata3/tfap2a/c quadruple morphants (4-MO). Arrows indicate relevant expression domains in placodal tissues. (A–C) Dorsal views (anterior up) of pitx3 expression in anterior pituitary and lens placode. (D–F) Lateral views (anterior to left) of foxe3 expression in the lens placode. (G–I) Frontal views of cxcr4b expression in olfactory placode. (J–L) Lateral views (anterior to left) showing the lens and nasal pits in live specimens at 30 hpf. Asterisks in (L) depict the absence of morphologically discernable structures. (M–O) Lateral views (anterior up) of isl1 expression in the trigeminal placode. (P–R) Lateral views (anterior up) of sox3 expression in the epibranchial placode. (S–U) Dorsal views (anterior up) of pax2a expression in the otic placode. (V–X) Dorsal views (anterior up) of cldna expression in the otic vesicle. All placodal markers are expressed normally in DM-treated embryos. Expression of cldna is severely deficient in quadruple morphants (X, n = 13/21) or ablated altogether (8/21, not shown). All other placodal markers are ablated in quadruple morphants (n≥10 for each marker). EXPRESSION / LABELING:
PHENOTYPE:
|
|
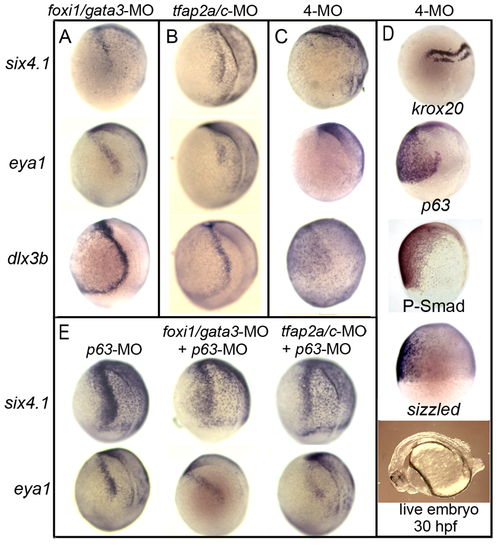
Knockdown of competence factors impairs preplacodal specification. (A–C) Expression of preplacodal markers at 10.5 hpf in (A) foxi1/gata3 double morphants, (B) tfap2a/c double morphants, (C) foxi1/gata3/tfap2a/c quadruple morphants (4-MO). Note the complete loss of preplacodal markers in C. (D) Expression of krox20, p63, P-smad and sizzled during gastrulation in foxi1/gata3/tfap2a/c quadruple morphants. Morphology of a live quadruple morphant at 30 hpf is also shown. (E) Expression of six4.1 and eya1 in p63 morphants alone or in combination with tfap2a/c-MO or foxi1/gata3-MO. All images show lateral views with dorsal to the right and anterior up, except for the live specimen in (D), which shows a lateral view with anterior to the left. EXPRESSION / LABELING:
|
|
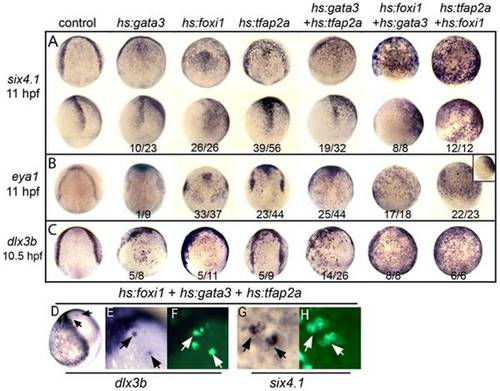
Misexpression of competence factors induces ectopic expression of preplacodal markers. (A–C) Analysis of indicated gene expression patterns in control embryos and embryos carrying hs:gata3, hs:foxi1 and/or hs:tfap2a heat-shocked at 30% epiboly (4.5 hpf). Dorsal views with anterior up except bottom row in A, inset in B, which are lateral views with dorsal to the right. Note the ectopic expression of PPE markers, six4.1 (A), eya1 (B) and dlx3b (C) in neuroectoderm of embryos misexpressing one or more competence factors. (D–H) Dorsolateral views (anterior up) of mosaic embryos showing ectopic expression of dlx3b and six4.1 at 10.5 hpf. Donor cells obtained from Tg(hs:foxi1) injected with hs:gata3 and hs:tfap2a plasmid were transplanted into wild type hosts and heat shocked at 4.5 hpf at 39°C. Transplanted cells were identified with Strepavidin-FITC (arrows F, H). Mosaic embryos shows cell autonomous expression of dlx3b and six4.1 in the neural plate (compare E, F and G, H). EXPRESSION / LABELING:
|
|
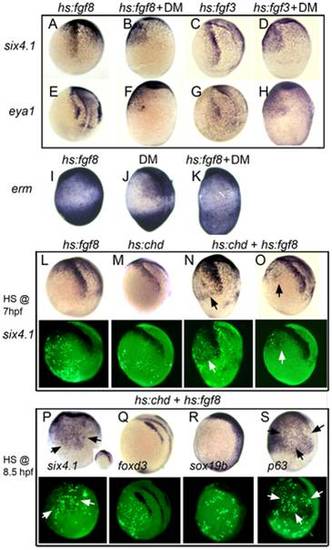
The entire nonneural ectoderm is competent to form preplacodal tissue. (A–H) Expression of preplacodal markers in (A, B, E, F) Tg(hs:fgf8) embryos incubated at 35°C from 7.5–10.5 hpf, or (C, D, G, H) Tg(hs:fgf3) embryos incubated at 36°C from 7–10.5 hpf. 100 µM DM was added as indicated. (I–K) Expression of erm in (I) Tg(hs:fgf8) embryo incubated at 35°C without DM, (J) a non-transgenic embryos incubated at 35°C with 100 µM DM, and (K) a Tg(hs:fgf8) embryo incubated at 35°C with 100 µM DM. (L–S) Mosaic misexpression of Fgf8 and/or Chordin. (L–O) Brightfield images (top row) and fluorescent images (bottom row) of host embryos with cells transplanted from Tg(hs:fgf8) (L), Tg(hs:chd) (M) or Tg(hs:fgf8); Tg(hs:chd) donor embryos (N, O). Donor embryos were injected with lineage tracer (biotin-dextran) and transplanted at mid-blastula (L, M, N) or early gastrula stage (O) into unlabeled host embryos. Embryos were heat-shocked at 39°C for 30 minutes at 7 hpf and examined for six4.1 expression at 10.5 hpf. Transplanted transgenic cells were identified by Strepavidin-FITC staining after in situ hybridization. All panels show lateral views of host embryos with anterior up. Mosaic embryos with Tg(hs:fgf8);Tg(hs:chd) double transgenic cells showed ectopic six4.1 expression in surrounding ventral ectoderm (N, O), whereas no ectopic six4.1 expression was detected following activation of hs:fgf8 alone (L) or hs:chd alone (M). (P–S) Brightfield images (top row) and fluorescent images (bottom row) of host embryos with cells transplanted during early gastrula stage from double heterozygous Tg(hs:fgf8); Tg(hs:chd) embryos. Embryos were heat shocked for 30 minutes at 39°C beginning at 8.5 hpf and examined for expression of six4.1 (preplacodal ectoderm), foxd3 (neural crest), sox19b (neural plate) or p63(epidermal ectoderm) at 10.5 hpf. All panels show lateral views except (P) which shows a ventral view (lateral view in inset) and (S) which shows ventro-lateral view. Heat shock activation at 8.5 hpf (P) leads to stronger ectopic expression of six4.1 than heat shock at 7 hpf (O). No ectopic expression of foxd3 or sox19b is detected (Q, R) whereas p63 expression appears downregulated in and around transgenic cells (arrows in S). EXPRESSION / LABELING:
|
|
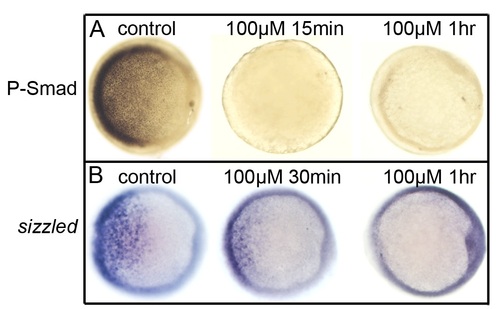
Dorsomorphin acts quickly to block Bmp signaling. Embryos were treated with either 1% DMSO (controls) or 100 µM DM beginning at 5 hpf. (A) Phospho-Smad staining in a control after 1 hour, or in DM-treated embryos after 15 minutes or 1 hour. (B) Expression of sizzled in a control embryo after 1 hour, or in DM-treated embryos after 30 minutes or 1 hour. All images show animal pole views with dorsal to the right. |
|
Additional data showing the effects of DM on preplacodal development and cell survival. (A, B) Expression of preplacodal markers at 10.5 hpf following addition of 100 µM DM at 5.5 hpf or 6 hpf. Treatment at 5.5 hpf eliminated expression of eya1 and six4.1, whereas dlx3b was either lost or expressed in bilateral stripes (the specimens processed for dlx3b expression were from the same experiment). Treatment at 6 hpf yielded two classes of embryos, with some showing loss of preplacodal markers and others showing bilateral stripes of preplacodal markers (the two specimens processed for six4.1 expression were from the same experiment). (C) Dorsal views of embryos stained with acridine orange (AO) at 11 hpf following addition of DMSO (control) or 200 µM DM at 7 hpf. AO staining in is comparable in controls and DM-treated embryos. At least 20 specimens were examined for each marker and time point. |
|
Assessment of general embryonic pattering following global misexpression of competence factors. Plasmid vectors for hs:tfap2a or hs:gata3 were injected into wild-type embryos or Tg(hs;foxi1) transgenic embryos, as indicated across the top of the Figure. Embryos were heat shocked at 4.5 hpf, including the non-transgenic controls. A–F, expression of various markers at the indicated times: (A–C) neurectodermal markers sox19b, krox20 and fgf3 [56], [57], (D) Fgf-target gene erm, (E) epidermal marker p63 [46], [47] and (F) Bmp target gene sizzled [34]. Misexpression of competence factors does not block Bmp or Fgf signaling nor general features of axial patterning, though embryos appear partially dorsalized. (G) AO staining in the respective transgenic carriers. hs:gata3 showed reduced cell death while other transgenes alone or in combination resulted in slightly increased cell death compared to control embryos. (H) Lateral views of live embryos at 28 hpf. A–C, E, and G show dorsal views of embryos, D shows dorsolateral views, and F and insets in E show lateral views. |
|
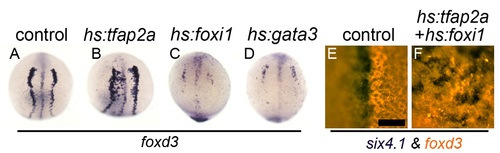
Effect of misexpression of competence factors on neural crest development. (A–D) Expression of foxd3 at 11 hpf in a control embryo (A), or following activation of hs:tfap2a (B), hs:foxi1 (C) or hs:gata3 (D) at 4.5 hpf. (E, F) Expression of six4.1 (blue) and foxd3 (red, fluorescence) in a control embryo (E) or following activation of hs:tfap2a and hs:foxi1 at 4.5 hpf (F). Scale bar = 50 µm. |
|
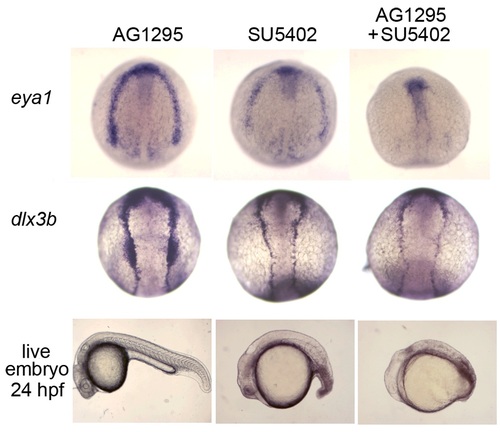
Blocking Fgf and Pdgf signaling leads to downregulation of preplacodal markers. (Upper two rows) Dorsal views showing expression of eya1 and dlx3b at 11 hpf in wild-type embryos that were treated beginning at 8.5 hpf with 15µM AG1295, 25µM SU5402, or both. AG1295 did not cause any significant changes in the expression. SU5402 reduced expression of both genes. Addition of both inhibitors caused loss of eya1 within the preplacodal domain and significant downregulation of dlx3b. (Lower row) Images of live embryos at 24 hpf. Treatment with SU5402 or both SU5402 and AG1295 severely perturbed caudal development and blocked formation of the otic vesicle. |
|
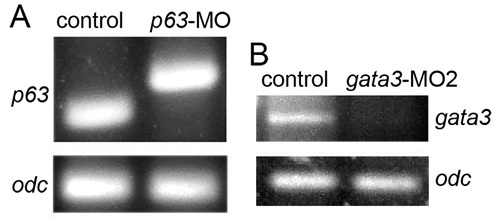
Effects of p63-MO and gata3-MO2 on accumulation of mature mRNA. (A) p63-MO leads to an aberrantly spliced transcript. Control embryos or embryos injected with p63 splice blocker were lysed at 11 hpf to collect mRNA. Primers for p63 and a constitutive control, ornithine decarboxylase (odc) were added to lysates to synthesize cDNA, which was then amplified for 30 cycles. p63-MO caused loss of wild-type transcript and accumulation of an aberrant splice product of higher molecular weight. (B) gata3-MO2 causes loss of gata3 transcript. Control embryos or embryos injected with gata3-MO2 (splice-blocker) were lysed at 12 hpf to collect mRNA. Primers for gata3 and odc were added to lysates to synthesize cDNA, which was then amplified for 30 cycles. Primers for gata3 flanked the splice junction between exons 1 and 2. Primer sequences: gata3: GTGTTGTGTGTATCGGTGAGTG, GAGGAGGAAGAAGCTGGAGG; odc: GGATGTCCTGAAGCACCT, CCCACTGACTGCACGAT; p63: Primers were the same as those used previously [63]. |