- Title
-
Fgf19 is required for zebrafish lens and retina development
- Authors
- Nakayama, Y., Miyake, A., Nakagawa, Y., Mido, T., Yoshikawa, M., Konishi, M., and Itoh, N.
- Source
- Full text @ Dev. Biol.
|
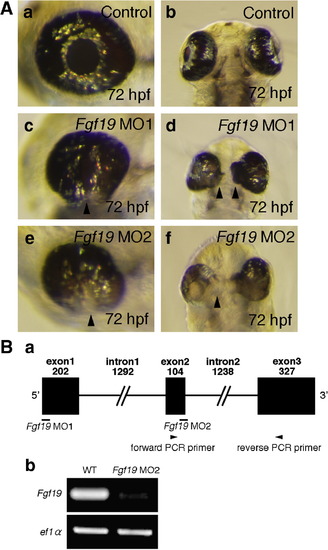
Morphology of the eye in Fgf19 MOs-injected embryos. A: Embryos were injected with control MO (a, b), Fgf19 MO1 (c, d), and Fgf19 MO2 (e, f). (a, b) In control MO-injected embryos, the eye developed normally. (c, d) Fgf19 MO1-injected embryos exhibited a significant reduction in the size of the lens and the retina at 72 hpf. (e, f) Fgf19 MO2-injected embryos also showed a significant reduction in the size of the eye, a failure of the choroid fissure to close, and the expansion of retinal tissue to the midline of the forebrain at 72 hpf. A failure of the choroid fissure to close, indicated by an arrowhead, was observed (c, e). A progressive expansion of retinal tissue to the midline of the forebrain, indicated by arrowheads, was also observed (d, f). (a, c, e) Lateral views with anterior to the left and dorsal to the top. (b, d, f) Ventral views with anterior to the top. B: (a) The coding region of Fgf19 was divided by two introns. Black boxes and black lines indicate exons and introns, respectively. Fgf19 MO1 anto the top. (b, d, f) Ventral views with anterior to the top. B: (a) The coding region of Fgf19 was divided by two intd Fgf19 MO2 indicate their target positions. Arrowheads indicate the positions of PCR primers for Fgf19 cDNA. (b) Fgf19 cDNA was amplified from wild-type or Fgf19 MO2-injected embryonic cDNA by RT-PCR using forward and reverse primers. ef1α cDNA was also amplified as a control. The cDNAs were analyzed by 1.5% agarose gel electrophoresis. After electrophoresis, the gel was stained with ethidium bromide. PHENOTYPE:
|
|
Expression patterns of Fgf19 in zebrafish embryonic eye. The expression patterns were determined by whole mount in situ hybridization. (a) Fgf19 was expressed in the future nasal retina at 18 hpf as indicated by an arrow. (b) By 20 hpf, the expression was confined to the anterior half of the neural retina as indicated by an arrow. Fgf19 expression in the lens was first detected at 20 hpf as indicated by an arrowhead. (c) Fgf19 expression intensified in the nasal retina and the lens at 27 hpf as indicated by an arrow and an arrowhead, respectively. (d) Fgf19 expression was detected in the inner region of the retina at 48 hpf as indicated by an arrow. (e, f) Fgf19 expression in the inner region of the retina was detected until 72 hpf as indicated by an arrow. (a–e) Lateral views with anterior to the left and dorsal to the top. (f) A transverse eye section at 72 hpf. EXPRESSION / LABELING:
|
|
Comparison of cell proliferation and cell death patterns in the lens and retina of control and Fgf19 MO1-injected embryos. A: Control and Fgf19 MO1-injected embryos were stained using an anti-H3P antibody. (a, b) Panels show representative transverse sections of the eye at 22 hpf. (c, d) The percentage of proliferating cells labeled with anti-phosphorylated Histone H3 antibody in the retina and lens of control and Fgf19 MO1-injected embryos. In the lens and retina, the injection of Fgf19 MO1 did not affect cell proliferation. B: Apoptotic cells in the eye of control and Fgf19 MO1-injected embryos were marked by TUNEL staining. (a, b) Panels show their representative transverse sections of the eye at 24 hpf. (c, d) The percentage of apoptotic cells in the retina and lens of control and Fgf19 MO1-injected embryos at 14, 18, 24, 36, 48 and 72 hpf. The ratio of the number of apoptotic cells to the total number of cells in the lens and retina increased significantly in Fgf19 MO1-injected embryos at 24 hpf. PHENOTYPE:
|
|
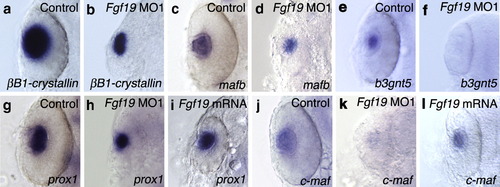
Expression of lens-specific marker genes at 24 hpf. Embryos were injected with control MO (a, c, e, g, j), Fgf19 MO1 (b, d, f, h, k) and Fgf19 mRNA (i, l). (a–d) The expression of βB1-crystallin (a, b) and mafb (c, d) was detected in the lens region of control MO-injected and Fgf19 MO1-injected embryos, while the expression domains in Fgf19 MO1-injected embryos were smaller than those in control embryos. (e, f) On the other hand, the expression of b3gnt5 was not detected in the lens region of Fgf19 MO1-injected embryos. (g, h) The expression of prox1 was detected in the lens region of control MO-injected and Fgf19 MO1-injected embryos. (i) In Fgf19 mRNA-injected embryos, the ectopic expression of prox1 was not detected. (j, k) In Fgf19 MO1-injected embryos, c-maf expression was lost in the lens fiber cells. (l) The ectopic expression of c-maf was not detected in Fgf19 mRNA-injected embryos. Dorsal views with anterior to the top. EXPRESSION / LABELING:
|
|
Neuronal differentiation and lamination of the retina in Fgf19 MO1-injected embryos. (a, b) The expression of neurod in control and Fgf19 MO1-injected embryos at 45 hpf. The expression of neurod in the outer retina was not affected in Fgf19 MO1-injected embryos. Lateral views with anterior to the left and dorsal to the top. (c, d) The expression of isl1 in control and Fgf19 MO1-injected embryos at 48 hpf. The expression of isl1 in the retina was not affected in Fgf19 MO1-injected embryos. Lateral views with anterior to the left and dorsal to the top. (e, f) The detection of HuC/D protein in control and Fgf19 MO1-injected embryos at 5 dpf. HuC/D was normally detected in the amacrine and ganglion cells of Fgf19 MO1-injected embryos. (g, h) Transverse eye sections of control and Fgf19 MO1-injected embryos at 3 dpf. The sections were stained with toluidine blue. The control retina showed a characteristic stratification into three nuclear layers and two plexiform layers. In Fgf19 MO1-injected embryos, the stratification of retinal layers occurred in the dorsal region, although the eye exhibited a reduction in size. However, the lamination of the ventral retina was abnormal in Fgf19 MO1-injected embryos. EXPRESSION / LABELING:
|
|
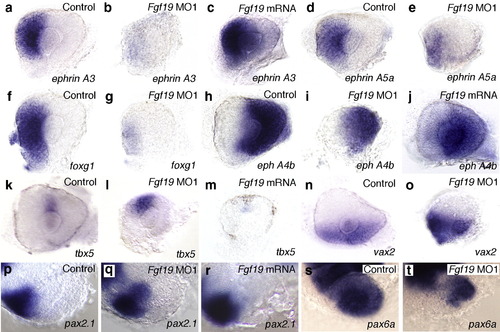
Nasal–temporal patterning of the retina in Fgf19 MO1-injected and Fgf19 mRNA-injected embryos. (a, d, f, h, k, n) Control embryos at 28 hpf. (b, e, g, i, l, o) Fgf19 MO1-injected embryos at 28 hpf. (c, j, m) Fgf19 mRNA-injected embryos at 28 hpf. (a-c) The expression of ephrin A3 was completely lost in the nasal half of the prospective retina of Fgf19 MO1-injected embryos, while the expression of ephrin A3 partially expanded into the temporal retina in Fgf19 mRNA-injected embryos. (d-g) The expression of ephrin A5a and foxg1 was severely reduced in the nasal retina of Fgf19 MO1-injected embryos. (h-j) The expression of eph A4b in the temporal half of the prospective retina partially expanded into the nasal retina in Fgf19 MO1-injected embryos, while the expression of eph A4b was decreased in the temporal retina of Fgf19 mRNA-injected embryos. (k, l) The nasal expansion of tbx5 expressetina in Fgf19 MO1-injected embryos, while the expression of eph A4b was decreased in the temporal retina of EXPRESSION / LABELING:
|
|
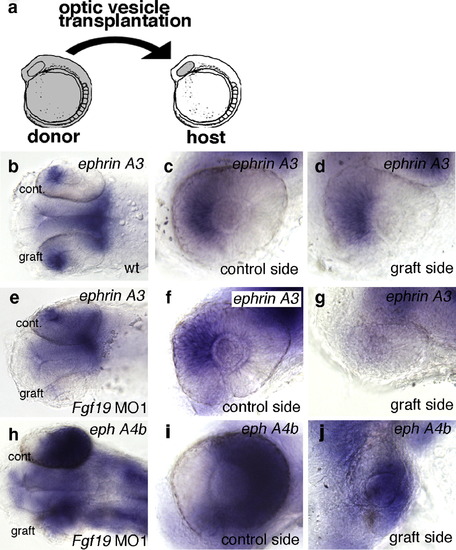
Nasal–temporal patterning of the retina after heterotopic optic vesicle transplantation. (a) Transplantation scheme: optic vesicles were grafted from wild-type donors to wild-type hosts, and from Fgf19 MO1-injected donors to wild-type hosts at the 10-somite stage. (b–j) Nasal–temporal patterning of the retina after transplantation at 28 h of development. (b–d) In optic vesicle grafts from wild-type embryos, the expression of ephrin A3 was correctly detected in the nasal retina. (e–g) In optic vesicle grafts from Fgf19 MO1-injected donors, the expression of ephrin A3 was lost in the nasal half. (h–j) In optic vesicle grafts from Fgf19 MO1-injected donors, the expression of eph A4b was slightly expanded into the nasal retina. (b, e, h) Dorsal views with anterior to the left. (c, d, f, g, i, j) Lateral views with anterior to the left and dorsal to the top. |
|
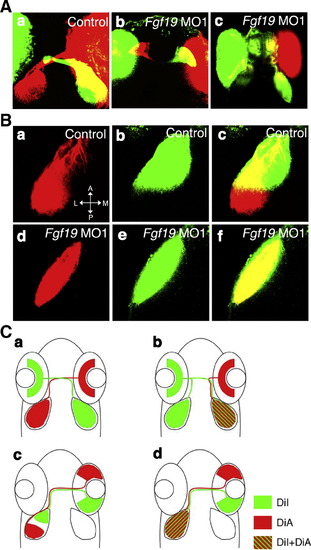
Guidance of retinal ganglion cell axons in Fgf19 MO1-injected embryos. A: Embryos were injected with control MO (a) and Fgf19 MO1 (b, c). (a) In control embryos, retinal axons projected to contralateral optic tecta. (b) The mild phenotype embryos showed normal projections. (c) In the severe phenotype embryos, retinal axons frequently projected to ipsilateral optic tecta or in some cases projected to both ipsilateral and contralateral optic tecta. B: Embryos were injected with control MO (a–c) and Fgf19 MO1 (d–f). Retinal ganglion cell axons anterogradely double-labeled by DiI (green) and DiA (red) in the dorsotemporal and dorsonasal quadrants. (a–c) In control embryos, nasal and temporal retinal ganglion cells projected to the posterior and anterior tectum, respectively. (d–f) Both nasal and temporal retinal ganglion cells projected to the entire tectum in Fgf19 MO1-injected embryos. C: Schematics of guidance of retinal ganglion cell axons in control (a, c) and Fgf19 MO1-injected (b, d) embryos. Dorsal views with anterior to the top. PHENOTYPE:
|
|
Interactions between Fgf3, Fgf8 and Fgf19 in the eye. (a, b) The expression of Fgf3 in control and Fgf19 MO1-injected embryos at 24 hpf. In control embryos, the expression of Fgf3 was detected in the optic stalk and the dorsal retina. The expression of Fgf3 was up-regulated in the retina of Fgf19 MO1-injected embryos. (c, d) The expression of Fgf8 in control and Fgf19 MO1-injected embryos at 24 hpf. In control embryos, the expression of Fgf8 was detected in the proximal retina. The ectopic expression of Fgf8 was detected in the nasal retina of Fgf19 MO1-injected embryos. (e, f) The expression of Fgf19 in wild-type embryos and embryos injected with both Fgf3 MO and Fgf8 MO at 24 hpf. In embryos injected with both Fgf3 MO and Fgf8 MO, Fgf19 expression in the inner retina was slightly decreased, while that in the lens was unaffected. Lateral views with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
|
|
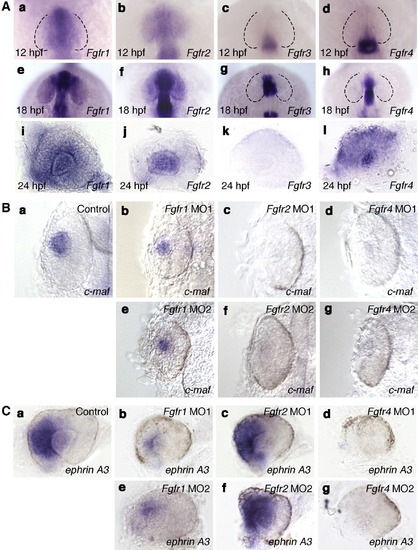
Expression pattern and function of Fgfrs in the eye. A: The expression patterns of Fgfr1 (a, e, i), Fgfr2 (b, f, j), Fgfr3 (c, g, k) and Fgfr4 (d, h, l) at 12 hpf (a–d), 18 hpf (e–h) and 24 hpf (i–l). (a, e) The expression of Fgfr1 was detected in the future nasal retina at 12 and 18 hpf. (i) At 24 hpf, the expression of Fgfr1 was detected in the lens and inner retina. (b) Fgfr2 expression was detected in the entire optic vesicle at 12 hpf. (f) At 18 hpf, Fgfr2 expression was detected in the lens. (j) By 24 hpf, Fgfr2 expression was detected at high levels in the lens. (c, g, k) The expression of Fgfr3 was not detected in the eye at 12, 18 and 24 hpf. (d, h) Fgfr4 expression was not detected in the eye at 12 and 18 hpf. (l) The expression of Fgfr4 was detected in the retina and lens at 24 hpf. (a–h) Dorsal views with anterior to the top. (i–l) Lateral views with anterior to the left and dorsal to the top. B: Expression of c-maf in wild-type embryos (a) and embryos injected with Fgfr1 MO1 (b) or MO2 (e), Fgfr2 MO1 (c) or MO2 (f) and Fgfr4 MO1 (d) or MO2 (g) at 24 hpf. The expression pattern of c-maf in the embryos injected with Fgfr1 MO1, Fgfr2 MO1 or Fgfr4 MO1 was essentially similar to that in embryos injected with Fgfr1 MO2, Fgfr2 MO2 or Fgfr4 MO2, respectively. (b, e) The expression of c-maf was reduced slightly in the lens region of Fgfr1 MO1- or MO2-injected embryos compared with wild-type embryos. (c, d, f, g) In the lens region of Fgfr2 MO1- or MO2-injected embryos or Fgfr4 MO1- or MO2-injected embryos, the expression of c-maf was not detected. Dorsal views with anterior to the top. C: Expression of ephrin A3 in wild-type embryos (a) and embryos injected with Fgfr1 MO1 (b) or MO2 (e), Fgfr2 MO1 (c) or MO2 (f) and Fgfr4 MO1 (d) or MO2 (g) at 28 hpf. The expression pattern of ephrin A3 in embryos injected with Fgfr1 MO1, Fgfr2 MO1 or Fgfr4 MO1 was essentially similar to that in embryos injected with Fgfr1 MO2, Fgfr2 MO2 and Fgfr4 MO2, respectively. (b, e) The expression of ephrin A3 was reduced in the nasal retina of Fgfr1 MO1- or MO2-injected embryos compared with control embryos. (c, f) In Fgfr2 MO1- or MO2-injected embryos, ephrin A3 expression was unaffected. (d, g) The expression of ephrin A3 was not detected in Fgfr4 MO1- or MO2-injected embryos. Lateral views with anterior to the left and dorsal to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
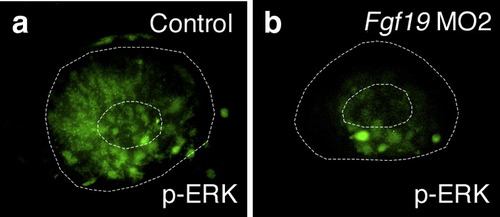
ERK activation in Fgf19 MO2-injected embryos. ERK1/2 activation was detected by whole mount immunohistochemistry in wild-type embryos (a) and embryos injected with Fgf19 MO2 (b) at 22 hpf. (a) In wild-type embryos, ERK1/2 activation was detected in the nasal retina and lens. (b) In Fgf19 MO2-injected embryos, ERK1/2 activation was suppressed in the nasal retina and lens. Lateral views with anterior to the left and dorsal to the top. |
Reprinted from Developmental Biology, 313(2), Nakayama, Y., Miyake, A., Nakagawa, Y., Mido, T., Yoshikawa, M., Konishi, M., and Itoh, N., Fgf19 is required for zebrafish lens and retina development, 752-766, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.