- Title
-
Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina
- Authors
- Bernardos, R.L., Lentz, S.I., Wolfe, M.S., and Raymond, P.A.
- Source
- Full text @ Dev. Biol.
|
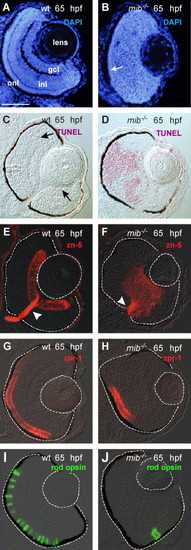
Differentiation and laminar organization of the retina in wild-type and mib-/- embryos at 65 hpf. DAPI-stained wild-type (A) and mib-/- (B) retinas. Lamination was absent in the mib-/- retina except for an outer nuclear layer (onl; arrow). TUNEL-labeled wild-type (C) and mib-/- (D) retinas (Fast Red—red). Representative apoptotic cells are indicated by arrows in the wild-type. Ganglion cells labeled with the zn-5/neurolin antibody in wild-type (E) and mib-/- (F) retinas (Cy-3—red; arrowhead = optic nerve; N = 10 embryos examined for each condition). (G–J) Opsin expression in wild-type and mib-/- retinas. Expression of red opsin (Cy-3—red) in wild-type (G) and mib-/- (H) retinas (N = 10 embryos). Expression of rod opsin (FITC—green) in rod photoreceptors in wild-type (I) and mib-/- (J) retinas (N = 10 embryos). Abbreviations: ganglion cell layer (gcl); inner nuclear layer (inl); outer nuclear layer (onl). Dorsal is up and ventral is down in this and all subsequent figures unless otherwise noted. The lens and outer border of the retina in this figure and all subsequent figures has been outlined to orient the reader. Scale bar = 50 μm (A–J). EXPRESSION / LABELING:
|
|
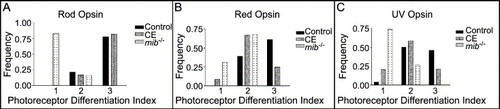
Frequency histograms of the photoreceptor differentiation index. Levels of photoreceptor differentiation, as measured by opsin expression at 65 hpf in control, mib-/- and CE-treated retinas (See text for a description of the index). (A) Rod photoreceptor differentiation progressed normally in CE-treated retinas and controls, but was significantly slowed in mib-/- retinas (Table 2). (B) Red cone differentiation was slowed in CE-treated and mib-/- retinas compared to controls (Table 2). (C) UV cone differentiation was also slowed in CE-treated and mib-/- retinas compared to controls (Table 2). |
|
Müller glia differentiation is inhibited in mib-/- retinas at 65 hpf. Müller glia in the retina (outlined) expressed zrf-1/GFAP (arrowhead) as did astrocytes and/or radial glia in the brain (*) of wild-type embryos (A). In contrast, zrf-1/GFAP expression was absent in the retina (outlined) but present in the brain (*) of mib-/- embryos (B; N = 10). (C, D) Expression of her6/hes1 in wild-type and mib-/- 65 retinas. (C′, D′) Differential interference contrast (DIC) images of the retinas in (C and D; N = 8 embryos). (C, C′) In the wild-type embryos, her6/hes1 expression was present in the brain (*) and in the germinal zone (arrowhead), lens epithelium (small arrow) and inner nuclear layer (large arrow) of the retina. (D, D′) The mib-/- embryos expressed her6/hes1 in the brain (*) but expression in the eye was restricted to the lens epithelium (small arrow). Scale bar = 50 μm (A–D). EXPRESSION / LABELING:
|
|
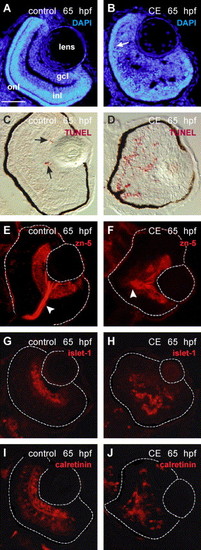
Differentiation and laminar organization of the retina in control and γ-secretase-inhibited (CE) embryos at 65 hpf. (A, B) Retinal cell nuclei labeled by DAPI (blue). The retinal cell layers are distinguishable in the control retina (A) but in the CE-treated retina only the onl (arrow) can be identified (B). TUNEL-labeled control (C) and CE-treated (D) retinal cryosections (Fast Red—red). The arrows in (C) point to apoptotic cells in the wild-type retina. Ganglion cells and their axons in the optic nerve (arrowhead) were labeled by zn-5/neurolin in control (E) and CE-treated (F) retinas (N = 12 embryos). Ganglion cell nuclei were labeled by islet-1 antibody in control (G) and CE-treated (H) retinas (N = 10 embryos). The retinal boundary is outlined. The calretinin antibody labeled ganglion, amacrine, and bipolar cells (based on location and morphology) in the retinas of control embryos (I) and neurons in the CE-treated embryos (J) (N = 8 embryos). Scale bar = 50 μm (A–J). Abbreviations as in Fig. 1. EXPRESSION / LABELING:
|
|
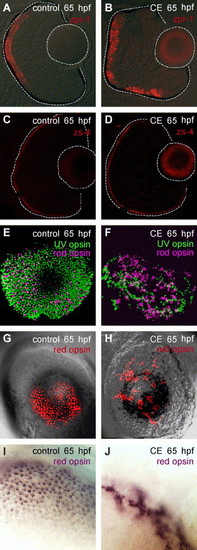
Photoreceptor differentiation and planar organization in control and γ-secretase-inhibited (CE) embryos. (A, B) Double-cones labeled by zpr-1 at 65 hpf (N = 15 embryos). (C, D) The outer limiting membrane (apical surface) was labeled by zs-4 (N = 15 embryos). (E–J) Whole-mount preparations of control and CE-treated eyes processed for in situ hybridization using opsin-specific riboprobes. Rod (Fast Red-pseudo-colored magenta) and UV (FITC-green) opsin in control (E) and CE-treated (F) retinas at 65 hpf. Red opsin (Fast Red-red) in control (G) and CE-treated (H) retinas at 65 hpf. Higher magnification of control (I) and CE-treated (J) retinas stained for red opsin expression (NBT/BCIP-purple). Scale bar = 50 μm (A-H); 20 μm (I, J). EXPRESSION / LABELING:
|
|
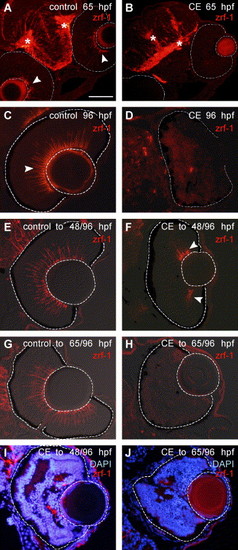
Müller glia fail to differentiate in the absence of Notch–Delta signaling. Immunostaining with zrf-1/GFAP (Cy3—red). In a control embryo at 65 hpf (A), radial glia in the brain are marked with an asterisk (*) and in the retina, endfeet of Müller glia at the vitreal surface are indicated by arrowheads. The apparent immunoreactivity in the lens is non-specific background. In an embryo treated in γ-secretase inhibitor (CE) up to 65 hpf (B), there is no staining with zrf-1/GFAP in the retina (outlined), but zrf-1/GFAP is present in the brain (*) (N = 20 embryos). At 96 hpf in a control retina (C) zrf-1/GFAP-labeled profiles (arrowhead) span the retina, whereas in a CE-treated embryo (D), there is no staining in the retina (outlined) (N = 20 embryos). (E, F) Retinas from embryos treated in control or drug solutions up to 48 hpf and then transferred to drug-free embryo media to continue development up to 96 hpf. In a CE-treated embryo (F) (N = 20 embryos examined), a few Müller glia (arrowheads) are indicated at the periphery of the retina (outlined) in contrast to the numerous Müller glia present in the control retina (E). (G, H) Retinas from embryos treated in control or drug solutions up to 65 hpf and then transferred to drug-free embryo media to continue development up to 96 hpf. In a drug-treated embryo (H), no zrf-1/GFAP staining is present in the retina (outlined) (N = 20 embryos). (I, J) Overlays of DAPI-stained and zrf-1/GFAP images show the relationship of Müller glia to the laminated regions of the retina in embryos treated with CE up to 48 hpf and then allowed to develop in drug-free embryo media up to 96 hpf (I) and embryos treated with CE up to 65 hpf and then allowed to develop in drug-free embryo media up to 96 hpf (J). Scale bar = 100 μm (A, B); 50 μm (C–J). |
|
Lack of her6/hes1 expression reflects inhibition of Notch signaling. Cryosections of control and γ-secretase-inhibited (CE) embryos labeled for her6/hes1 expression (FITC—green) by in situ hybridization. DIC images indicate the location of her6/hes1 expression in the retina (outlined) in the paired panels to the left. (A, A′) At 65 hpf, her6/hes1 expression in the inl (large arrow) and germinal zone (arrowhead) of the retina and in the lens epithelium (small arrow) in control embryos. (B, B′) her6/hes1 expression in the lens epithelium (small arrows) and brain (*) of CE-treated embryos (N = 15 embryos). (C, C′) At 96 hpf, her6/hes1 is expressed in the inl, often in radial streaks (large arrow), and in the germinal zone (arrowhead) of the retina and in the lens epithelium (small arrow) of control embryos (N = 15 embryos). A faint reaction product is also seen in the gcl, but this may be localized to the endfeet of Müller glia—a similar pattern of gfap mRNA expression is observed in retinas at this stage (R.L.B., unpublished observations). (D, D2) In embryos treated with CE up to 48 hpf and then allowed to develop in drug-free embryo media up to 96 hpf, her6/hes1 is expressed in the inl (large arrow), germinal zone of the retina (arrowhead), the optic nerve head (*; astrocytes) and in the lens epithelium (small arrow). Co-labeling with zrf-1/GFAP (Cy-3-pseudo-colored magenta) reveals a correlation between the location of zrf-1/GFAP and her6/hes1 expression in the inl (N = 15 embryos). (E, E2) In embryos treated with CE up to 65 hpf and then allowed to develop in drug-free embryo media up to 96 hpf, her6/hes1 is expressed only in the retinal germinal zone (arrowhead) and lens epithelium (small arrows) and no zrf-1/GFAP immunoreactivity is detected in the retina (N = 15 embryos). Scale bar = 50 μm (A–E). |
|
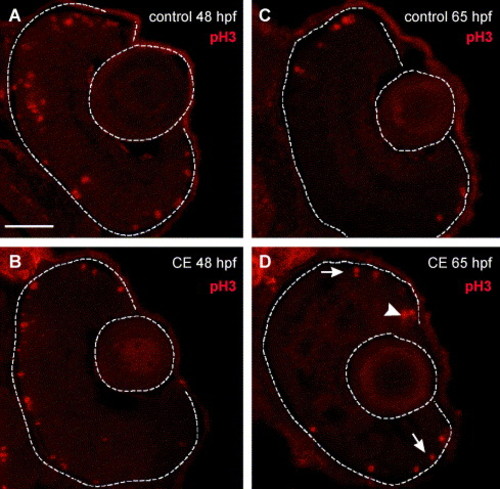
Mitotic activity in the retinas of control and γ-secretase-inhibited (CE) embryos. (A–D) Retinal cryosections labeled with phosphorylated histone H3 (pH3) antibody (Cy3—red). (A) Control and (B) CE-treated retinas labeled with anti-pH3 at 48 hpf. Mitotic cells were present in the outer retina in control and drug-treated retinas. By 65 hpf, mitotic activity was restricted to the germinal zone at the ciliary margin (arrow) and outer nuclear layer (arrowheads) in control (C) and CE-treated (D). Scale bar = 50 μm (A–D). |
|
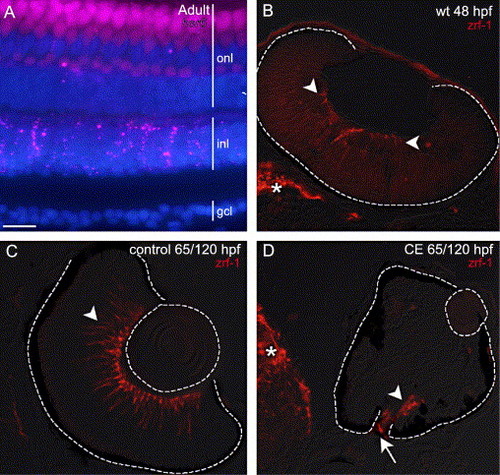
(A) Expression of her6/hes1 (pseudo-colored magenta) in an adult wild-type zebrafish retina co-labeled with DAPI (blue). her6/hes1 expression is present in the inner nuclear layer (inl), and a few cells in the outer nuclear layer (onl), but is absent from the ganglion cell layer (gcl). The apparent labeling in the onl is due to photoreceptor autofluorescence. (B) Expression of zrf-1/GFAP in the retina (arrowheads) and brain (*) of a wild-type embryo at 48 hpf. The earliest expression of GFAP in zebrafish Müller glia reported previously was 5 dpf (Scheer et al., 2001). (C, D) Retinas from embryos treated in control and CE solutions up to 65 hpf and then transferred to drug-free embryo media to continue development up to 120 hpf. At 120 hpf in a control retina (C) zrf-1/GFAP-labeled profiles (arrowhead) span the retina, whereas in a γ-secretase-inhibited (CE) embryo (D), there is no staining in the inner retina, but staining is present (arrowhead) at the optic disc (arrow) and in the brain (*) (N = 15 embryos). Scale bar = 15 μm (A), 50 μm (C, D) or 30 μm (B). |

Unillustrated author statements |
Reprinted from Developmental Biology, 278(2), Bernardos, R.L., Lentz, S.I., Wolfe, M.S., and Raymond, P.A., Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina, 381-395, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.