- Title
-
UXT potentiates angiogenesis by attenuating Notch signaling
- Authors
- Zhou, Y., Ge, R., Wang, R., Liu, F., Huang, Y., Liu, H., Hao, Y., Zhou, Q., Wang, C.
- Source
- Full text @ Development
|
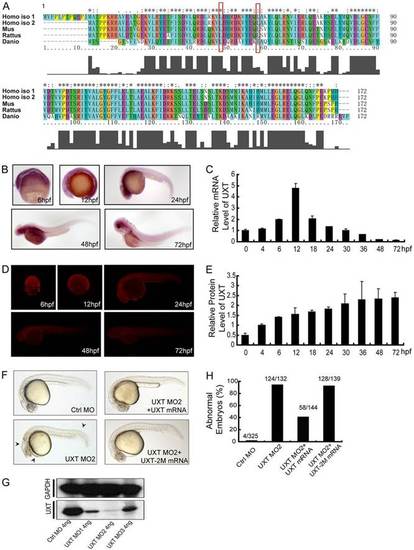
UXT is evolutionarily conserved and developmentally expressed. (A) A sequence alignment of human, mouse, rat and zebrafish UXT proteins by Clustal X. The histogram below the ruler indicates the degree of similarity. Peaks indicate positions of high similarity and valleys indicate low similarity. The asterisk indicates positions that have been fully conserved. The red rectangles indicate the conserved amino acid sites for UXT-2M mutation. (B) Whole-mount in situ hybridization of UXT in zebrafish embryos from 6hpf to 72hpf. The developmental time points are indicated in the panels. (C) Quantification of the UXT mRNA expression in zebrafish embryos from 6hpf to 72hpf by using RT-qPCR. Data show the mean±s.e.m. (at least three independent experiments). (D) Antibody staining of UXT in zebrafish embryos from 6hpf to 72hpf. (E) Quantification of UXT protein expression in zebrafish embryos with the UXT-specific antibody 4B4, from 0hpf to 72hpf, normalized to GAPDH. Densitometry was performed using ImageJ. Data show the mean±s.e.m. (at least three independent experiments). (F) Phenotypic analyses of zebrafish embryos at 24hpf. Embryos were injected with 4ng of control morpholino (Ctrl MO), 4ng of UXT MO2 or 4ng of UXT MO2 with 150pg of UXT mRNA (UXT MO2+UXT mRNA). Additional embryos were injected with 4ng of UXT MO2 plus 150pg of UXT mRNA mutant (UXT MO2+UXT-2M mRNA). All embryos were analyzed at 24hpf. The morphological defects are indicated by black arrowheads. (G) The knockdown efficiency of the morpholinos. Embryos were injected with 4ng of control morpholino, 4ng of UXT MO1, 4ng of UXT MO2 or 4ng of UXT MO3. The embryos were harvested at 24hpf and the lysates were probed with the anti-zebrafish UXT-specific antibody 4B4. (H) Graphical representation of zebrafish phenotypic analyses; the number of embryos analyzed in each group is indicated above the bars. EXPRESSION / LABELING:
PHENOTYPE:
|
|
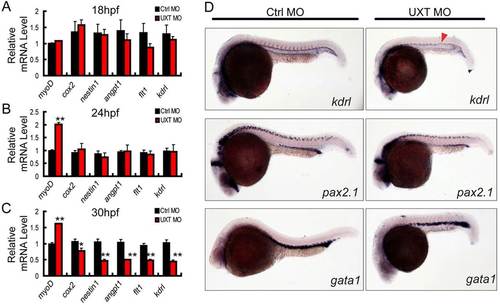
UXT is essential for zebrafish angiogenesis. (A-C) RT-PCR verification of angiogenesis markers in 18hpf, 24hpf or 30hpf zebrafish embryos injected with 4ng of control morpholino (Ctrl MO) or with 4ng of UXT morpholino (UXT MO). Data shown are the mean±s.e.m. (at least three independent experiments); *P<0.05, **P<0.01 versus the corresponding control. (D) Whole-mount in situ hybridization of embryos injected with 4ng of control morpholino (Ctrl MO) or 4ng of UXT morpholino (UXT MO). Riboprobes against the angiogenesis marker kdrl, the pronephric marker pax2.1 and the erythroid marker gata1 were visualized at 22hpf. The red arrowhead indicates the decreased expression of kdrl in UXT-deficient embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
|
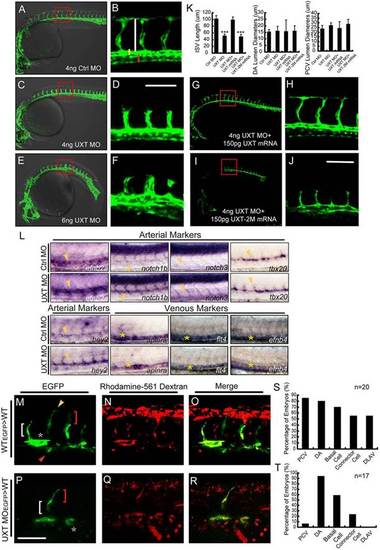
UXT is indispensable for zebrafish ISV spouting. (A-F) Confocal imaging of the UXT-deficient Tg(kdrl:EGFP)s843 embryos at 30hpf. (A,C,E) Bright-field images merged with confocal images. (B,D,F) Higher magnification images of the confocal-imaged area in the red boxed regions in A,C and E. (A) General morphology and trunk vascular phenotype and (B) the ISV sprouts in a Tg(kdrl:EGFP)s843 embryo injected with 4ng of control morpholino (Ctrl MO). (C) General morphology and trunk vascular phenotype, and (D) the ISV sprouts in a Tg(kdrl:EGFP)s843 embryo injected with 4ng of UXT morpholino (UXT MO). (E) General morphology and trunk vascular phenotype, and (F) the ISV sprouts in a Tg(kdrl:EGFP)s843 embryo injected with 6ng of UXT MO. The ISVs are marked by the vertical white line. The yellow and red bars indicate the lumen of the dorsal aorta (DA) and posterior cardinal vein (PCV), respectively. All of the embryos were confocal imaged at 30hpf and are shown in lateral views with rostral left and dorsal up. Scale bar:100µm. (G-J) Confocal imaging of the Tg(kdrl:EGFP)s843 morphants rescued by UXT mRNA. (H,J) The confocal imaged area of the red boxed regions in G and I. (G) The trunk vascular phenotype and (H) the ISV sprouts in a Tg(kdrl:EGFP)s843 embryo injected with 4ng of UXT morpholino plus 150pg of wild-type UXT mRNA (4ng UXT MO+150pg UXT mRNA). (I) The trunk vascular phenotype and (J) the ISV sprouts in a Tg(kdrl:EGFP)s843 embryo injected with 4ng of UXT MO plus mutant UXT mRNA (4ng UXT MO+150pg UXT-2M mRNA). All of the embryos were confocal imaged at 30hpf. Scale bar: 100µm. (K) Statistical analysis of the ISV length and the lumen diameter of the DA or PCV from embryos injected with Ctrl MO, UXT MO, UXT MO+UXT mRNA or UXT MO+UXT-2M mRNA. For one experiment, six embryos of each treatment were analyzed at 30hpf. Data show the mean±s.e.m. (at least three independent experiments); ***P<0.001 versus the corresponding control. (L) The whole-mount in situ hybridization of UXT-deficient embryos at 30hpf. Riboprobes are against the arterial markers efn2a, notch1, notch3, tbx20, hey2 and the venous markers aplnra, flt4 and efnb4. The yellow arrowheads denote the DA; the yellow asterisks indicate the PCV. (M-R) Confocal imaging of the donor Tg(kdrl:EGFP)s843 cells in non-transgenic embryos at 30hpf. (M) Wild-type cells contribute to all trunk vessels, including the DA (asterisk), PCV (red arrowhead), basal cells (red bracket), connector cells (white bracket) and DLAV (yellow arrowhead). (P) Deficient for UXT, the endothelial cells of Tg(kdrl:EGFP)s843 can only contribute to theDA, basal cells and connector cells. (N,Q) The transplanted donor cells were stained with Rhodamine-561 dextran. (O,R) Merged images of EGFP and Rhodamine-561 dextran. Scale bar: 75µm. (S,T) Quantification of wild-type (S) and UXT-deficient (T) donor Tg(kdrl:EGFP)s843 cells in non-transgenic embryos. n, the number of successfully transplanted embryos. |
|
UXT modulates endothelial cell migration and cell cycle. (A,B) Confocal time-lapse images (from 19hpf to 30hpf) of Tg(kdrl:EGFP)s843 embryos injected with 4ng of control MO (A) or 4ng of UXT MO (B). The numbering on the images shows the indicated time points. Scale bars: 50µm. (C) Quantification of endothelial cell number in the segmental artery sprouts. (D) Quantification of the migration speed of ISV tip cells in control embryos and UXT morphants. (E,F) The inhibitory effect of UXT on cell cycle. (E) Flow cytometric analysis for BrdU incorporation and 7-AAD labeling in wild-type and UXT-deficient HUVECs or UXT-deficient HUVECs treated with 1.5µM DAPT. (F) Quantification of the percentage of cells in different cell cycle stages. All quantitative data are the mean±s.e.m. (at least three independent experiments);*P<0.05, **P<0.01, ***P<0.001 versus the corresponding control. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
UXT interacts with NICD endogenously through the Notch TAD domain. (A) UXT interacts with NICD endogenously. HUVEC cell lysates were immunoprecipitated (IP) with an anti-Notch1 antibody or control IgG antibody. The immunoprecipitates were immunoblotted (IB) with the indicated antibodies. (B) UXT directly attenuates Notch-targeted genes in endothelial cells. The relative expression level of hes1 and hey1 were measured by RT-PCR. The data were normalized on the basis of the corresponding input control, and are presented as the mean±s.e.m. (at least three independent experiments). (C) UXT attenuates Notch signaling in vivo. Confocal images of Tg(TP1:mCherry; fli1:EGFP)y1 fish injected with 4ng of control MO or 4ng of UXT MO. (D) UXT interacts specifically with NICD. HA-UXT or HA-UXT-2M was co-transfected with Flag-NICD or Flag-RBP-JK. Cell lysates were subjected to an immunoprecipitation assay using the anti-Flag antibody, followed by western blot analysis with anti-HA and anti-Flag antibodies. (E) UXT impairs the interaction between NICD and RBP-JK. HUVECs were treated with shUXT or phage-UXT-Flag. The endogenous NICD was immunoprecipitated with the anti-Notch1 antibody, and the immunoprecipitates were probed with the anti-RBP-JK antibody. (F) Schematic representation of the NICD deletion constructs used in the following experiments. (G) The TAD domain of NICD mediates its interaction with UXT. HA-UXT was co-transfected with ΔRAM-Flag, ΔANK-Flag, ΔTAD-Flag, ΔPEST-Flag or NICD-Flag. Cell lysates were subjected to an immunoprecipitation assay using an anti-Flag antibody. (H) UXT directly impaired Notch signaling. The indicated plasmids were transfected into Cos-7 cells together with TP-1 reporter plasmids, in the presence of NICD or the deletion mutants. Data are presented as the mean±s.e.m. (n=3); **P<0.01; ***P<0.001; ns, non-significant versus the corresponding control or as indicated. |
|
UXT potentiates angiogenesis through Notch signaling. (A-C) UXT potentiates angiogenesis in vitro. (A) Knocking down UXT by shRNAs or ectopically expressing wild-type or mutant UXT (phage plasmids) was performed in HUVECs, followed by the in vitro angiogenesis assay. Fluorescence images were taken (A) and quantified (B,C). Scale bar: 500µm. Relative mean mesh area (B) and relative branching length (C) were calculated by using Image J. (D-F) 3D in vitro angiogenesis with collagen gel-embedded cytodex beads coated with HUVECs, treated with shNT, shUXT, phage-vector or phage-UXT. Cumulative length and the numbers of all sprouts originating from an individual cytodex bead were quantified using ImageJ after 24h, with ten cytodex beads analyzed per experimental group. Scale bar: 200 µm. (G-I) Fluorescence images (G) and quantification (H,I) of the in vitro angiogenesis assay on HUVECs treated with DMSO or DAPT. Wild-type (shNT) or UXT-deficient (shUXT) HUVECs were seeded on Matrigel and then treated with either DMSO or DAPT (1.5µM) for 6h. Scale bar: 500µm. Relative mean mesh area (H) and relative branching length (I) were calculated using ImageJ. The data were normalized on the basis of the corresponding input control. (J-Q) Tg(kdrl:EGFP)s843 embryos were treated with either 60µM DAPT or DMSO. (J,K) Tg(kdrl:EGFP)s843 embryos injected with 4ng of control morpholino (Ctrl MO) were treated with DMSO. (N,O) Tg(kdrl:EGFP)s843 embryos injected with 4ng of control morpholino were treated with DAPT. (L,M) Tg(kdrl:EGFP)s843 embryos injected with 4ng of UXT morpholino (UXT MO) were treated with DMSO. (P,Q) Tg(kdrl:EGFP)s843 embryos injected with 4ng of UXT morpholino were treated with DAPT. All images were taken at 30hpf. The confocal images in K,O,M,Q show higher magnification of areas of the bright-field images to the left. The red arrowheads indicate the robust angiogenesis in ISVs, whereas the yellow arrowheads highlight the DLAV growth rescued by DAPT treatment. Scale bar: 100µm. (R-U) 2ng of RBP-JK morpholino were injected into Tg(kdrl:EGFP)s843 embryos without (R,S) or with (T,U) 4ng of UXT morpholino. All the images were taken at 30hpf. The confocal images in S,U show higher magnification of areas of the bright-field images to the left. Red arrowheads indicate excessive angiogenesis in ISVs, and yellow arrowheads denote DLAV growth rescued by co-injection of the RBP-JK morpholino. Scale bar: 100µm. (V) Quantification of J-U. The ISV branching points per segment were analyzed. Five different embryos were analyzed per experimental group. The data were normalized on the basis of the corresponding input control. All quantitative data are presented as the mean±s.e.m. (at least three independent experiments); *P<0.05, **P<0.01, ***P<0.001 versus the corresponding control. |