- Title
-
Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish
- Authors
- Carreira-Barbosa, F., Concha, M.L., Takeuchi, M., Ueno, N., Wilson, S.W., and Tada, M.
- Source
- Full text @ Development
|
pk1 is expressed maternally and in migrating mesodermal precursors in zebrafish. (A) Homology of Prickle proteins between zebrafish (Pk1), Xenopus (XPk-A) (Wallingford et al., 2002b) and human (BAB71198). The numbers refer to the percentage aminoacid identities between the different orthologues. (B-E) Expression of pk1 at early stages. pk1 is expressed maternally at the eight-cell stage (B, animal view), on the dorsal side at ∼40% epiboly (C, lateral view with dorsal to the right), and around the germ ring at 50% epiboly (D, animal view with dorsal to the right). At 90% epiboly (E, dorsal view, F, lateral view, anterior is up), expression is in dorsal involuted cells and in overlying ectodermal cells and highlighted in the axial cells (arrowheads). At tailbud stage (G, dorsal view, anterior is up), expression is restricted to the presomitic mesoderm, posterior neuroectoderm, the lateral edge of neural plate (arrows) and anterior axial mesodermal cells (black arrowhead), but slightly downregulated in the posterior axial mesodermal cells (red arrowhead). d, dorsal; s, shield. |
|
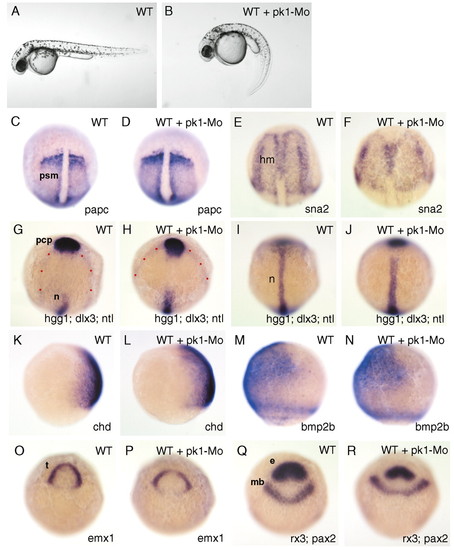
pk1 morphants exhibit defects in convergent extension movements. (A,B) Lateral views of pharyngula stage living wild-type (A) and pk1-morphant (B) embryos. The pk1-morphant embryo (B) shows a slightly compressed trunk with a curled down tail. (C-J,O-R) Dorsal views of tailbud-stage wild-type (WT) and pk1-morphant (WT + pk1-Mo) embryos. (K-N) Lateral views of 80% epiboly wild-type (WT) and pk1-morphant (WT + pk1-Mo) embryos. Anterior is up and genes analysed are indicated bottom right. Dots outline the prospective neural plate. e, eye field; mb, prospective midbrain; t, prospective telencephalon; psm, presomitic mesoderm; hm, head mesoderm; n, prospective notochord, pcp, prechordal plate. EXPRESSION / LABELING:
|
|
Abrogation of Pk1 function enhances the slb phenotype and suppresses elevated Wnt11 activity. (A-D) Dorsal views showing prechordal plate position (arrowheads) in a slb-/- embryo (A), a slb-/- embryo injected with 3 ng of pk1-Mo (B), a slb-/- embryo injected with 10 pg wnt11 RNA (C) and a slb-/- embryo injected with 10 pg wnt11 RNA plus 3 ng of pk1-Mo (D). Dots outline the prospective neural plate. The positions of the prechordal plate are indicated by arrowheads. |
|
Abrogation of Pk1 function enhances the ppt phenotype. Lateral (A,C,E,G) and dorsal (B,D,F,H) views of 14-somite embryos with anterior to the left. myoD expression shows the shape of the somites in (B,D,F,H). Arrowheads indicate the most anterior and posterior extent of the axis. (A,B) Wild-type embryos. (C,D) Wild-type embryos injected with 3 ng of pk1-Mo. (E,F) ppt-/- embryos. (G,H) ppt-/- embryos injected with 3 ng of pk1-Mo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
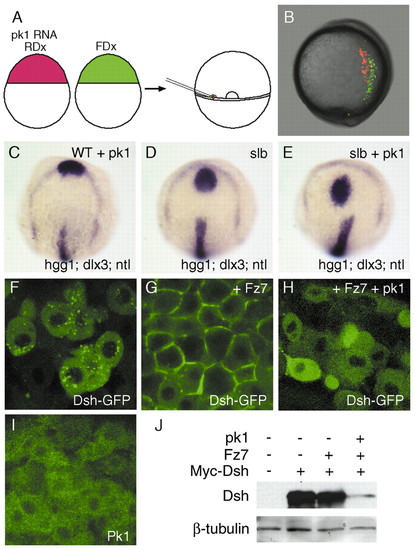
Overexpression of pk1 causes defective morphogenetic movements and enhances the slb phenotype. (A) Schematic of the experiment. Cells from embryos that received 5 pg pk1 RNA and rhodamin-dextran (RDx) and cells from embryos that received fluorescein-dextran (FDx) were transplanted simultaneously into the germ rings of wild-type, shield-stage hosts. (B) Lateral view of a living, tailbud-stage host embryo with dorsal to the right. Pk1-expressing cells (red) are positioned more laterally compared to wild-type cells (green). (C-E) Gain-of-function of pk1 enhances the slb phenotype. Dorsal views of a tailbud-stage embryo injected with 0.5 pg of pk1 RNA (C), a slb-/- embryo (D) and a slb-/- embryo injected with 0.5 pg pk1 RNA (E). The position and shape of prechordal plate (hgg1) in relation to the anterior edge of neural plate (dlx3) in the pk1 morphant (C) are similar to wild-type (see Fig. 2I). The prechordal plate is displaced caudally in the slb-/- embryo (D) and even further caudally in the slb-/- embryo injected with the pk1-Mo (E). (F-H) Confocal images of animal pole cells of embryos at 40% epiboly injected with 200 pg RNA encoding Dsh-GFP (F), 200 pg RNA encoding Dsh-GFP and 50 pg fz7 RNA (G) and 200 pg RNA encoding Dsh-GFP, 50 pg fz7RNA and 5 pg pk1 RNA (H). Dsh preferentially localises to vesicles (F) but relocates to the membrane in the presence of Fz7 (G). The Fz7-induced membrane localisation of Dsh is inhibited by Pk1 (H). (I) Subcellular localisation of Pk1 in animal-pole cells of 40% epiboly embryos injected with 25 pg RNA encoding Venus-Pk1 (Pk1 tagged with a modified version of EGFP). Pk1 localises in the cytoplasm. (J) Western-blot analysis of the levels of tagged-Dsh in embryos injected with 200 pg RNA encoding myc-Dsh along with 50 pg fz7 RNA in the presence or absence of 5 pg pk1 RNA. The blot was detected with either an anti-myc antibody for Dsh or an anti-β-tubulin antibody for loading control. The level of Dsh is reduced by pk1. |
|
pk1 and tri show a strong genetic interaction in regulating convergent extension. Lateral views of pharyngula-stage embryos (A,C,E,G,I) and dorsal views of 14-somite embryos (B,D,F,H,J) with anterior to the left. myoD expression shows the shape of the somites and krox20 the position of rhombomeres (r3 and r5) in (B,D,F,H,J). (A,B) Wild-type embryos. (C,D) Wild-type embryos injected with 3 ng of pk1-Mo. (E,F) tri heterozygous embryos injected with 3 ng of pk1-Mo. (G,H) tri homozygous embryos. (I,J) tri homozygous embryos injected with 3 ng of pk1-Mo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Pk1 functions with Tri to mediate tangential neuronal migration. Dorsal views of hindbrains of embryos in which an isl1-GFP transgene labels cranial motor neurons (brown). Anterior to the left. Stage is indicated bottom left. (A,B) Expression of pk1 (blue) in relation to motor neurons (brown). The arrowheads indicate newly born (A) and migrating (B) facial motor neurons. pk1 is expressed at high levels anterior and posterior to the domain of facial motor neuron migration. In this domain, pk1 is expressed weakly in the vicinity of the migrating cells. (C-F) Wild-type embryo (C), embryo injected with 3 ng of pk1-Mo (D), tri heterozygous embryo (E) and tri heterozygous embryo injected with 1 ng of pk1-Mo (F). Migration of facial motor neurons (arrowheads) is disrupted in the embryo with severe abrogation of Pk1 function (D) and in the embryo with partial loss of Tri and Pk1 function (F). (G-J) Dorsal views of hindbrains of wild-type embryos (G,I) and of embryos injected with 3 ng of pk1-Mo (H,J). ot, otic vesicle; r3-5, rhombomeres 3-5. EXPRESSION / LABELING:
PHENOTYPE:
|