- Title
-
The transcriptional repressor REST/NRSF modulates hedgehog signaling
- Authors
- Gates, K.P., Mentzer, L., Karlstrom, R.O., and Sirotkin, H.I.
- Source
- Full text @ Dev. Biol.
|
Expression of rest during early zebrafish development. mRNA in situ hybridization with antisense rest probe, except H′ where rest sense strand was used. Wholemounts (A–C, H) and transverse sections (D–G, I–L) of wild-type embryos during the first 42 h of development. (A, B, D) Early expression is ubiquitous. (C–G) Expression remains widespread at 22 hpf (C) and 25 hpf (H) but transverse sections of hindbrain reveal progressive downregulation as neurogenesis takes place in ventrolateral domains (arrowheads, E–G). (I–L) Sections of 42 hpf embryo, taken at levels indicated in (H). The pattern of rest expression is similar along the anterior–posterior axis. rest is expressed in mitotic cells of the ventricular zone, (marked by white dashed line) and undifferentiated neural structures such as the eyes (I, J), and optic tectum (OT, J). (J–L) Most tissue outside the neural tube still expresses rest at this stage, such as the head mesenchyme (hm J, K), developing fins (L) endodermal tissue (end, L) neural crest (nc, L) and ectoderm surrounding the somites (ect, L) but not the the already differentiated somites (L, so) and sensory cranial ganglia (cg, K). cg, cranial ganglia; ect, ectoderm; end, endoderm; hm, head mesenchyme; OT, optic tectum; tg, tegmentum. |
|
The neural tube is ventralized in Rest knockdown embryos. Transverse sections of 29hpf (A, B) or 26hpf (C–H) wild-type embryos processed for RNA in situ hybridization and sectioned at the level of the hindbrain at anterior rhombomere 4. Control (A, C, E, G) and rest MO injected (B, D, F, H) embryos stained with antisense probes for Hh response genes pax3a, nkx2.2a, foxA2 and ptc1. pax3a expression (A, B) is reduced in rest morphants, while expression of nkx2.2a (C, D), foxA2 (E, F) and ptc1 (G, H) is expanded compared to stage matched control embryos. This suggests that Rest represses Hh signaling. Arrows (E, F) mark pharyngeal endoderm. (I) qPCR analysis on 29hpf control (red bars) and stage matched rest morphant (blue bars) cDNA for markers shown in A–H, plus class I gene dbx1a. Overall levels of class II Hh target genes nkx2.2a, foxA2, and ptc1 are increased while class I genes pax3a and dbx1a levels are reduced. |
|
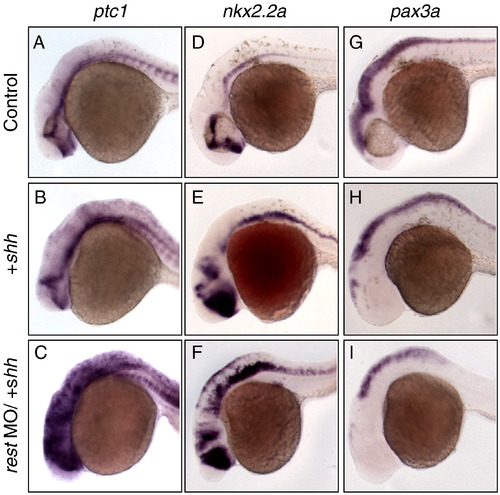
Rest knockdown sensitizes embryos to exogenous shh. Lateral, head and hindbrain views of 28 hpf control (A, D, G), shh mRNA (B, E, H) and shh mRNA/REST mo (C, F, I) microinjected embryos stained with antisense probes for Hh response genes. Injection of shh mRNA results in enhancement of ptc1 (B) and nkx2.2a (E), while pax3a (H) expression is reduced. rest morphants treated with the same amount of shh mRNA have increased expression of Hh target genes compared to shh mRNA treated embryos. These embryos have enhanced expression of ptc1 (C) and nkx2.2a (F), while pax3a is further reduced (I). This demonstrates that Rest knockdown enhances the response to high levels of Hh. EXPRESSION / LABELING:
|
|
CyA mediated attenuation of Hh signaling is enhanced by Rest knockdown. Lateral views of 26 hpf control (A, C, E, G) and rest morphants (B, D, F, H) embryos stained for nkx2.2a (A–D) or pax3a (E–H). Embryos were incubated in control media (A, B, E, F) or 1.5 μM cyclopamine (CyA) media from 6 hpf on (C, D, G, H). rest morphants incubated in control media show a modest increase in nkx2.2a expression (B, compare to control, A) and a modest decrease in pax3a (F, compare to control, E) grown under the same conditions. 1.5 μM CyA decreases nkx2.2a expression (C) and modestly expands pax3a (G) in control embryos. Rest knockdown produces a greater reduction in nkx2.2a expression, and a greater expansion of pax3a, then CyA treatment alone. EXPRESSION / LABELING:
|
|
Rest acts downstream of Smo in the Hh pathway. Lateral views of 24 hpf embryos injected with control MO (A, E) rest MO (B, F), dominant-negative PKA mRNA (dnPKA, C, G), or both (D, H) and stained for nkx2.2a. Embryos were placed in control media (A–D) or media containing 50 μM cyclopamine (E–H). Injection of dnPKA mRNA expands nkx2.2a expression (C). This expansion is augmented by co-injection with rest MO (D). CyA treatment partially attenuates the effects of dnPKA mRNA injection on nkx2.2a expression (G). dnPKA mRNA/rest mo injected embryos (F) are more resistant to CyA treatment then dnPKA treated embryos. EXPRESSION / LABELING:
|
|
Rest represses gli2a expression. Transverse sections of the hindbrain of control (A, C, E, G) and stage-matched rest morphants (B, D, F, H). RNA in situ hybridization to monitor gli1 (A, B) gli3 (C, D) and gli2a (E–H) expression. (A, B) gli1 expression is enhanced by Rest knockdown. (C, D) gli3 expression is unaltered by Rest knockdown. (E, G) gli2a expression is downregulated ventrally and in the midline ventricular zone (vz) as development proceeds in control embryos. (F, H) gli2a expression is maintained in the vz and is expressed more ventrally in rest morphants. EXPRESSION / LABELING:
|
|
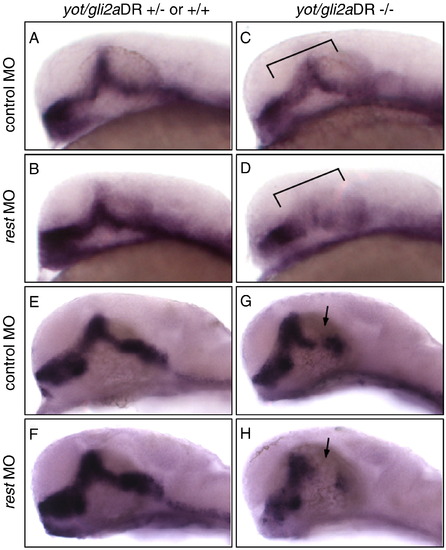
Rest knockdown in yot/gli2a mutants represses Hh target gene expression. Lateral view, 30 hpf embryos stained for ptc1 (A–D) or nkx2.2 (E–H) mRNA. rest knockdown in wild-type embryos (B, F) results in modest enhancement of ptc1 and nkx2.2a expression (compare B with A, and F with E). yot/gli2a mutants show characteristic loss of ptc1 (C) and nkx2.2a (G). In yot mutants with compromised Rest function (D, H), the loss of ptc1 (D) and nkx2.2a (H) expression is more pronounced in the midbrain/diencephanlon region (indicated by brackets, D and arrows, H) than in control-injected mutants (C, G). EXPRESSION / LABELING:
|
|
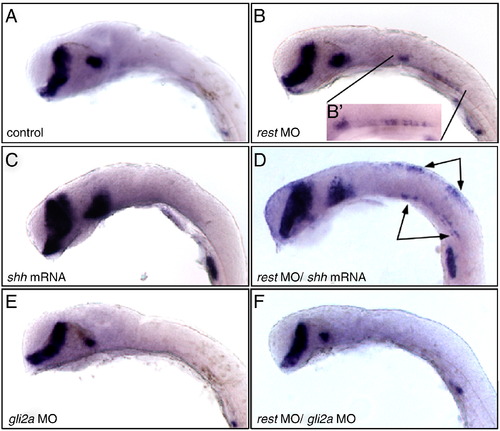
Rest mediated repression of Hh target gene expression in gli mutants requires Gli2a. dtr/gli1 mutants, lateral views, head and hindbrain, stained for nkx2.2a by whole mount RNA in situ hybridization. Embryos were injected with control MO (A), rest MO (B), shh mRNA (C), rest mo/shh mRNA (D), gli2a MO (E) or rest MO/gli2a MO (F). In dtr/gli1 mutants, nkx2.2a expression is absent in control-injected embryos (A). However, hindbrain expression is partially restored in dtr/gli1 mutants treated with rest morpholino (arrows, B). dtr mutants are largely refractory to exogenous shh mRNA (C) dtr/gli1 mutants treated with rest morpholino have a qualitatively different response to shh mRNA treatment (D). Gli2a knockdown results in decreased midbrain nkx2.2a expression (E) The restoration of hindbrain nkx2.2a expression in dtr mutants produced by Rest knockdown (arrows, inset in B) is eliminated by simultaneous knockdown of Gli2a (compare F with B). This reveals that the enhancement of Hh signaling produced by Rest knockdown requires Gli2a and Gli1. EXPRESSION / LABELING:
|
|
rest MO effectively reduces wild-type rest transcript levels. (A) Diagram of the rest premRNA showing the oligonucleotides and morpholino used for these experiments. rest splice blocking morpholino (MO) binds the intron 2-exon3 boundary of zebrafish rest and is predicted to result in the inclusion of intron 2. Primers (arrows) that amplify a 130 bp region spanning exon 2 and exon 3 in the wildtype mRNA are separated by 3,674 bp after morpholino treatment if intron 2 is not removed. This product is not amplified under the PCR conditions used. (B) Real Time PCR analysis of rest transcript levels at 6 hpf, 12 hpf and 24 hpf after micro-injection of control or rest mo. Wild-type rest mRNA is reduced following treatment with rest morpholino (C) RT-PCR of 24hpf control or rest morphant cDNA using primers designed to amplify a 173 bp mRNA product containing intron 2. The PCR product predicted to be produced as a result of the morpholino treatment is enriched in rest morphant cDNA. Note: a similar product is present at low levels in control and may represent a splice variant (Rest4) that is present in other species. |
|
Rest represses nkx2.2a expression in pancreas. Lateral view at anterior trunk level of de-yolked embryos stained with antisense probe for nkx2.2a. Pancreas (arrows) is more prominent in stage matched rest morphants (B) than in embryos injected with control mo (A) EXPRESSION / LABELING:
|
|
Rest knockdown enhances the effects of ectopic Hh signaling. Lateral views of embryos injected with control MO (Left column), shh mRNA (middle column) or shh mRNA in conjunction with rest MO (right column). (A-U) Embryos fixed around 24-28hpf and stained for Hh response genes . (V-X) Live 32hpf transgenic islet:eGFP embryos. (Middle column) Injection of shh mRNA results in dorsal expansion (B, E, H, K, N, W) or reduction (Q, T) in displayed markers. (Right column) Co-injection of rest MO alongside shh mRNA causes greater dorsal expansion (C, F, I, L,O, X) and a greater reduction in genes negatively regulated by Hh signaling (R, U). |
|
rest morphant phenotypes are specific to Rest knockdown. Lateral views, 1dpf embryos stained with nkx2.2a (A-D) or pax 3 antisense riboprobes (E-H). Hindbrain, anterior trunk views of embryos treated with control MO (A, C) or translation blocking rest ATG MO (B, D) and co-injected with shh mRNA (C, D) rest ATG MO sensitizes embryos to ectopic Hh signaling, (compare D to C). This phenocopies the effects of the rest splice blocking MO. (E-H) Injection of shh mRNA represses pax3 expression (F, compare with E). The heightened effect of exogenous Shh in rest morphants (G, compare with F) is reversed by rest mRNA (H, compare with G). EXPRESSION / LABELING:
|
|
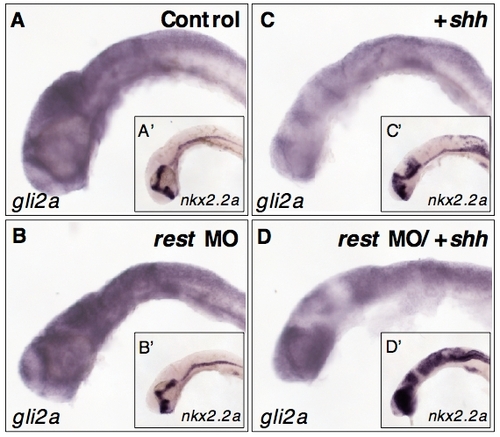
Hh-induced downregulation of gli2a is not enhanced by co-injection of rest MO. Lateral view, head and hindbrain of embryos probed for either gli2a (A-D) or nkx2.2a (inserts A′-D′) for comparison. (A, B) gli2a expression in control and age matched rest morphants. C) Overexpression of shh results in decreased levels of gli2a and increased levels of nkx2.2a (C′). (D) Embryos injected with both rest mo and shh mRNA have dramatically enhanced nkx2.2a expression (D′), co-injection does not correspondingly downregulate gli2a expression (D). EXPRESSION / LABELING:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 340(2), Gates, K.P., Mentzer, L., Karlstrom, R.O., and Sirotkin, H.I., The transcriptional repressor REST/NRSF modulates hedgehog signaling, 293-305, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.