- Title
-
Endoglin is a conserved regulator of vasculogenesis in zebrafish-Implications for hereditary haemorrhagic telangiectasia
- Authors
- Zhang, D., Zhou, F., Zhao, X., Liu, B., Chen, J., Yang, J.
- Source
- Full text @ Biosci. Rep.
|
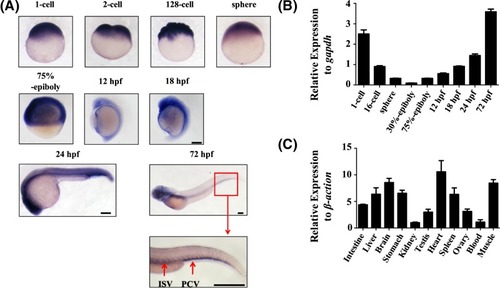
Temporal-spatial expression of endoglin ( |
|
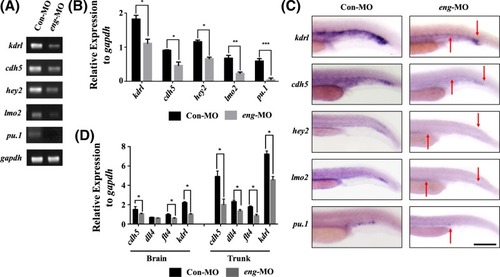
Endoglin knockdown affected the development of the vasculature ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
Endoglin knockdown decreased the expression of endothelial markers ( |
|
Bmper was involved in endoglin-regulated vasculogenesis ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
Enhancing the expression of BMPER increased the expression of ID1 and blood vessel formation in ( |