- Title
-
belladonna/(lhx2) is required for neural patterning and midline axon guidance in the zebrafish forebrain
- Authors
- Seth, A., Culverwell, J., Walkowicz, M., Toro, S., Rick, J.M., Neuhauss, S.C., Varga, Z.M., and Karlstrom, R.O.
- Source
- Full text @ Development
|
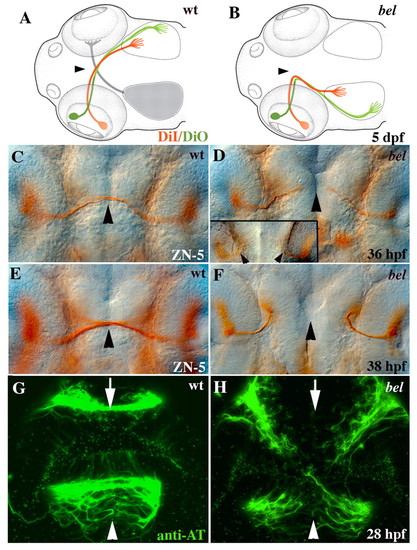
Retinal and commissural axon defects in bel. (A) Schematic showing that all retinal axons cross the wild-type midline (arrowhead) and project topographically on the contralateral tectal lobe [anterior/dorsal retina (green) to posterior/ventral tectum, posterior/ventral retina (red) to anterior/dorsal tectum]. (B) In bel mutants, retinal axons fail to cross the midline (arrowhead) and instead project to the ipsilateral tectal lobe in a topographically correct manner (see Karlstrom et al., 1996). (C) At 36 hpf, wild-type RGC axons have reached the midline (arrowhead). (D) In most bel mutants, RGC axons have grown towards the midline (arrowhead), but in some mutants axons project ipsilaterally immediately after leaving the eye (inset, arrowheads). (E) At 38 hpf, wild-type RGC axons have crossed the midline (arrowhead). (F) In bel mutants, all RGCs project ipsilaterally at 38 hpf. (G) Both the AC (arrow) and POC (arrowhead) are fully formed in wild-type embryos by 28 hpf. (H) In bel mutants, both forebrain commissures fail to form. (A,B) Schematic dorsal views of the head, anterior left (adapted, with permission, from Culverwell and Karlstrom, 2002). (C-H) Ventral views of the forebrain, eyes on either side of each panel, anterior up. |
|
Embryonic expression of zebrafish lhx2. (A) At 23 hpf, lhx2 is expressed in most of the telencephalon, anterior diencephalon and in the epiphysis. Inset shows lhx2 expression in the anterior CNS at the tailbud stage (11 hpf, arrowhead). (B) At 26 hpf, lhx2 is regionally expressed in the telencephalon, diencephalon and epiphysis. Inset shows reduced lhx2 expression only in the dorsal/anterior diencephalon in a bel mutant (arrowhead). (C,D) lhx2 continues to be regionally expressed in the forebrain, midbrain and hindbrain at 32 and 48 hpf. By 48 hpf, lhx2 expression is reduced at the chiasm region and also in the telencephalon. (E) Lateral view shows lhx2 expression in the midbrain/hindbrain border and hindbrain at 32 hpf (arrowheads). (F) Dorsal view shows lhx2 expression at rhombomere boundaries (arrowheads) at 48 hpf. (G) lhx2 expression throughout the eye field (arrowhead) at 15 hpf. Initial expression in the eye field is low relative to that in the forebrain. (H) At 21 hpf, lhx2 is expressed throughout the optic vesicle, with higher levels of expression in the dorsonasal region (arrowhead). (I) At 32 hpf and 48 hpf (inset), lhx2 is expressed in the marginal zones (arrowheads) and in the amacrine cell layer (arrow) of the eye. (J) By 3 dpf, expression in the amacrine layer becomes restricted ventrally (arrow). (K,L) lhx2 is expressed in most of the fin bud at 26 hpf (K) and becomes restricted to the posterior bud at 48 hpf (L, arrowheads). (A-E) Lateral views, anterior to the left; (F,G,J,K) dorsal views; (H,I) 7 μm sections of the eye. Black dots mark the position of the optic recess. e, epiphysis; di, diencephalon; hb, hindbrain; mb, midbrain; mhb, midbrain-hindbrain boundary; t, telencephalon. EXPRESSION / LABELING:
|
|
Regulation of lhx2 expression. (A,B) Wild-type embryos were treated with SU5402 (B) or DMSO carrier (A) from 6 hpf to 10 hpf and labeled to show lhx2 expression. Blocking Fgf signaling during the onset of lhx2 expression nearly eliminated lhx2 expression in the embryo (B, arrowhead). Insets show complete loss of the Fgf-regulated gene erm in the forebrain and hindbrain of identically treated embryos (arrows). (C,D) Treating embryos with SU5402 from 10 hpf to 24 hpf reduced lhx2 expression in the telencephalon (arrowheads) and diencephalon (arrow). All panels show lateral views of the head, anterior to the left. Eyes were removed in C and D. |
|
Forebrain patterning defects in bel mutants. (A,B) In bel mutants (B), dlx2 expression is extremely reduced in anterior/dorsal diencephalon (arrows) and is slightly reduced in the telencephalon (brackets), compared with in wild type (A). Inset in A shows a similar loss of dlx2 expression in the anterior diencephalon after injection of lhx2 antisense MO into a wild-type embryo. (C,D) In bel mutants (D), nk2.1b expression is regionally disrupted in the diencephalon (arrows) and expanded at a small region at the optic recess (arrowhead), compared with in wild type (C). E, F) fgf8 expression is extremely reduced in the optic stalk region (arrowheads) in bel mutants (F), compared with wild type (E). Insets show anterior views of the head, with fgf8 expression absent at the midline (arrowheads) in bel mutants. (G, H) In bel mutants (H), pax2.1 expression is absent in the medial optic stalk (arrowheads) but remains laterally. (I,J) zic2.1 expression is similarly absent across the midline (arrowheads) in bel mutants (J). (K, L) The number of phospho-histone-labeled mitotic cells is reduced in the dorsal/anterior diencephalon of bel mutants (brackets). (M) Graph showing a significant reduction in the number of proliferating cells in the mutant diencephalon (asterisk, P<0.01). (N, O) Anterior views showing the loss of dlx2 expression in the anterior diencephalon of bel mutants (arrows, compare with lateral views in A,B). (P-U) Fluorescein dextran-labeled cells (red/brown) were transplanted between wild-type and bel mutant embryos. (P,R,T) Transplanted wild-type cells express dlx2 (blue) in a wild-type (P) and bel mutant (R,T) background (seven embryos). (Q) Transplanted bel mutant cells do not express dlx2 in the ventral midline in bel mutants. (S,U) In a wild-type background (19 embryos), bel mutant cell clones do not express dlx2, but populate the midline and are intermingled with wild-type cell clones. (T,U) Higher magnification view of transplanted cells in the diencephalon. (T) dlx2 transcripts (blue) and lineage tracer (red) are present in wild-type cells (purple arrowheads) in a bel mutant forebrain. (U) By contrast, alternating blue (dlx2-positive wild type) and red (lineage tracer containing dlx2-negative bel mutant) cells occupy the ventral midline in a wild-type background (red and blue arrowheads). (A-F,K,L) Lateral views of the forebrain, anterior left, eyes removed; (G-J) ventral views of the forebrain, anterior up; (N-U) anterior views of the forebrain, dorsal up; (T,U) Black dots mark the position of the optic recess. Scale bars: 15 μm. |
|
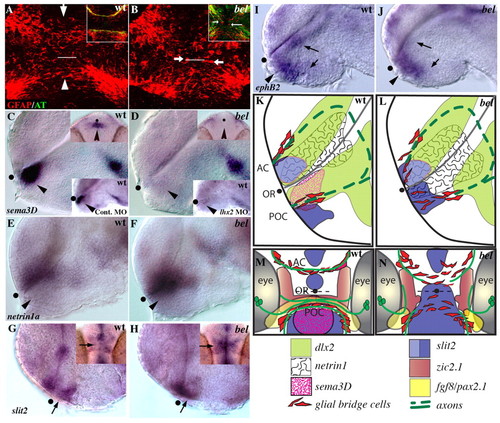
The axon growth substrate is disrupted in bel mutants. (A, B) Confocal images of ventral views of 21 hpf embryos labeled with anti-GFAP antibody (red); white line marks the optic recess. (A) GFAP expressing glial cells span the midline in the position of the AC (arrow) and POC (arrowhead). (B) In bel mutants, these glial bridges are disorganized and glial cell bodies are present between the commissures (arrows). Insets show a similar disorganization of glial cells in the mutant at 28 hpf in embryos double labeled for axons (anti-AT, green) and glial cells (anti-GFAP, red). (C, D) sema3d is absent in the forebrain of the bel mutants (arrowhead). Upper insets show ventral views; lower insets show a similar loss of sema3d expression in the anterior diencephalon after injection of lhx2 antisense MO into a wild-type embryo. (E, F) netrin1a expression is expanded ventrally into the diencephalon in bel mutants (arrowhead). (G, H) Expression of slit2 in bel mutants is expanded across the chiasm region (arrows). Insets show ventral views, anterior up. (I, J) ephB2 expression is reduced in the diencephalon and hypothalamus (arrows), but appears normal in the chiasm region (arrowheads). (K-N) Schematic lateral (K,L) and ventral (M,N) views showing expression of a subset of the genes described in this and the previous figure. AC, anterior commissure; OR, optic recess; POC, post optic commissure; black dots mark the position of the optic recess. EXPRESSION / LABELING:
|
|
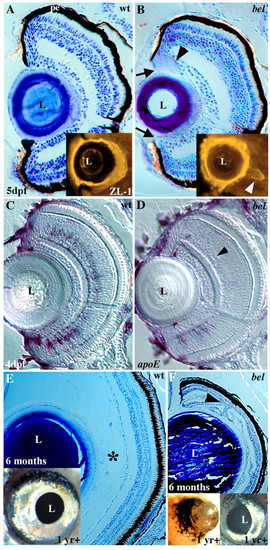
Larval and adult eye defects in bel mutants. (A, B) Toluidine Blue stained sections from 5 dpf wild type (A) and bel mutants (B). In bel mutants there is a gap between the PE and lens (arrows), and an abnormal acellular mass is often present (arrowhead). (Inset) In a different embryo, an acellular aggregate was labeled with the ZL-1 antibody, indicating it contains lens proteins (arrowhead). (C,D) At 4 dpf, bel mutants (D) lack most amacrine cells, as determined by apoE in situ labeling (arrowhead). A few cells remain in the ventral retina. (E, F) Eyes of surviving bel adults (F) are much smaller than those of wild type (E) due to the lack of a posterior compartment (asterisk). Retinal layers are folded back on one another (arrowhead). Insets show lateral views of whole eyes. (F, left inset) In some adults, vascularized retinal tissue extends out of the eye. L, lens; pe, pigmented epithelium. |

Unillustrated author statements |