- Title
-
An essential role for heat shock transcription factor binding protein 1 (HSBP1) during early embryonic development
- Authors
- Eroglu, B., Min, J.N., Zhang, Y., Szurek, E., Moskophidis, D., Eroglu, A., and Mivechi, N.F.
- Source
- Full text @ Dev. Biol.
|
HSBP1-MO-treated zebrafish embryos exhibit increased cellular domains expressing markers representing NC inducers. In situ hybridization analyses showing the expression of the indicated genes in the embryos at 12 hpf stage. (A) Expression of NC inducers in control MO- and HSBP1 MO1 and MO2-injected embryos. In all panels, dorsal views of the embryos show expression of Tfap2a, Snail2 or Foxd3. A total of 2000 embryos were injected and 100 embryos were used per probe for the in situ hybridization analyses. No nonspecific hybridization was observed with sense probes (data not shown). Morphant phenotypes were more than 90%, n=40. Mag. 40×. (B) Quantification of the expression of NC inducers in control MO- and HSBP1 MO1-injected embryos. For data quantification, photos were subdivided and the number of squares was quantified in both Y and X-axis. In the lower panels, numbers in the X-axis correspond to the numbers in the upper panels. EXPRESSION / LABELING:
PHENOTYPE:
|
|
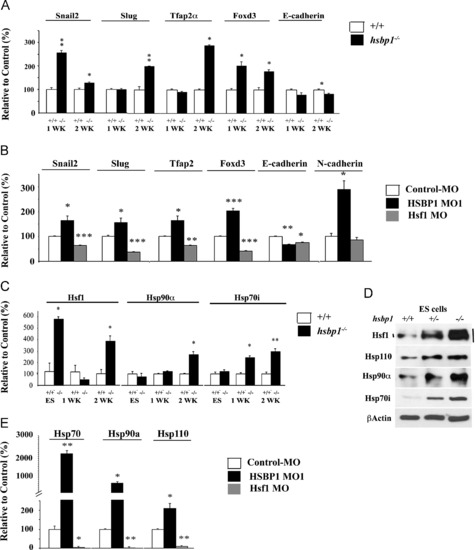
Gene expression profile of NC-inducers and Hsps in mouse EBs and zebrafish embryos. (A) Total RNA was isolated from one- and two-week-old EBs, and cDNAs were subjected to qPCR for the indicated genes. (B) Total RNA was isolated from the control MO, HSBP1 MO or Hsf1 MO-treated embryos at 12 hpf, and the cDNAs were subjected to qPCR for the indicated genes. (C) Total RNA was isolated from ES cells or one- or two-week old EBs and the cDNAs were subjected to qPCR for the indicated genes. (D) ES cell lysates from hsbp1+/+, +/- and -/- were subjected to immunoblotting to examine the levels of the indicated proteins. Hsf1-retarded mobility shifts indicate its increased activity. (E) Total RNA was isolated from the control MO, HSBP1 MO or Hsf1 MO -treated embryos at 12 hpf, and the cDNAs were subjected to qPCR for the indicated genes. In all panels, bars are mean +/- SEM. Statistical analyses were performed using Student t-tests. *p<0.05; **p<0.01 and ***p<0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
HSBP1 is evolutionarily conserved and is expressed during early embryonic development in zebrafish. (A) Human (hhsbp1), mouse (mhsbp1), rat (rhsbp1), Xenopus (xhsbp1), and zebrafish (zhsbp1) HSBP1 amino acids were aligned using CLUSTALW (http://pbil.ibcp.fr/htm/index.php). Conserved amino acids are highlighted in black. (B) mRNA and cDNA were prepared from zebrafish embryos at different stages of development. Semiquantitative RT-PCR was performed using hsbp1 specific primers. β-Actin was used as a control. Negative control contained no input mRNA. (C) Whole mount in situ hybridization was performed to detect HSBP1 expression in wild-type zebrafish embryos collected at the 8-cell stage, and then at 3, 6, 10, 16 and 24 hpf. HSBP1 is strongly expressed maternally. During somitogenesis (16 hpf), tail shows reduced expression, and at 24 hpf the expression is limited to the head region. Mag. 40x. EXPRESSION / LABELING:
|
|
HSBP1 morpholino (MO)-treated zebrafish embryos exhibit developmental defects. (A) One to two-cell-stage embryos were injected with control MO, HSBP1 MO1 or HSBP MO2. Embryos were photographed showing lateral views at different stages post MO-treatment. HSBP1 MO1 and MO2- injected embryos displayed anterior defects in the head region and developmental problems such as shortened body length and curved body axis. The HSBP1 MO1 or HSBP1 MO2-injected embryos were also co-injected with p53 MO to determine if HSBP1 4 MO-injected defects observed at 24 hpf or 48hpf can be partially rescued. The MO-treated morphant phenotypes were more than 80-85% (n=50). Mag. 40x. Percent defects for the embryos that survived at 24 hpf and 48 hpf are indicated in Table S2. (B) 24 hpf embryos injected with control MO (left panel); HSBP1 MO1 (middle panel); note that HSBP1 MO1 (right panel) and rescued with 500pg/embryo of HSBP1 mRNA. Arrowheads point to the embryos showing proper head region in control MO and HSBP1 MO1/HSBP1 mRNA groups and apoptotic head region in HSBP1 MO1-treated embryos. (C) One to two-cell-stage embryos were injected with control MO or HSBP1 MO2. At 24 hpf, embryo cell lysates were used in immunoblotting analyses using HSBP1 antibody. Lower panel shows Coomassie Blue staining as control for loading. In each experiment more than 300 embryos were injected and experiments were repeated 3 times. |
|
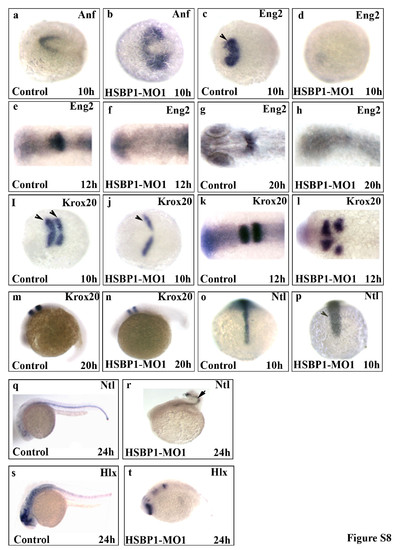
HSBP1 morpholino-injected embryos show aberrant expression of genes involved in neurodevelopment. In situ hybridization analyses showing the expression of the indicated markers in control MO-injected or HSBP1 MO-injected embryos at the indicated times post fertilization. The markers were as follows: Anf (a-b), Eng2 (c-h), Krox-20 (i-n), Ntl (o-r), and Hlx1 (s-t). Morphant phenotypes were more than 90%. n=40. Embryos are dorsal view (a-I; o-p) or lateral view (m-n; q-t). For in situ hybridization analyses a total of 3000 embryos were injected and 200 embryos were used per probe. No nonspecific hybridization was observed with sense probes (data not shown). Embryos were photographed using a SPOT camera mounted on a dissecting microscope. Mag. 40x. PHENOTYPE:
|
|
Phenotypes of the surviving MO-injected zebrafish embryos. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 386(2), Eroglu, B., Min, J.N., Zhang, Y., Szurek, E., Moskophidis, D., Eroglu, A., and Mivechi, N.F., An essential role for heat shock transcription factor binding protein 1 (HSBP1) during early embryonic development, 448-460, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.