- Title
-
Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent
- Authors
- Sun, S.K., Dee, C.T., Tripathi, V.B., Rengifo, A., Hirst, C.S., and Scotting, P.J.
- Source
- Full text @ Dev. Biol.
|
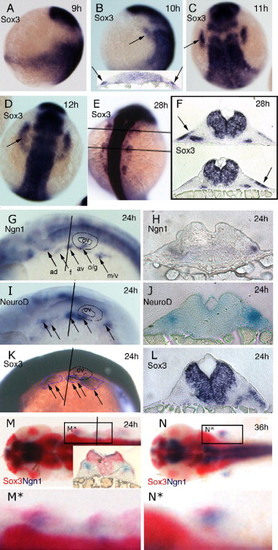
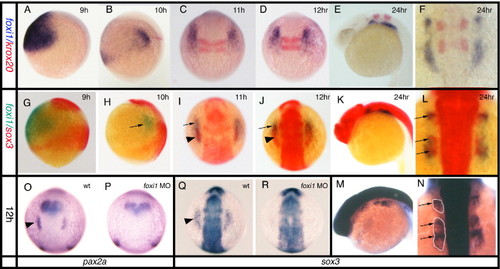
Expression of sox3 marks the developing epibranchial placodes. (A, B, G, I, K) Embryos viewed laterally with dorsal to right (A, B) or dorsal uppermost (G, I, K). (C–E, M, N) Embryos viewed dorsally with rostral uppermost (C–E) or to the left (M, N). In situ hybridization for sox3 expression shows its appearance in a small bilateral patch (arrow) of ectoderm (see insert in panel B showing t.s. section) either side of the hindbrain between 9 and 10 hpf (sox3 is also strongly expressed throughout the length of the CNS) (A, B). By 11 hpf (2–3 somites) these patches have formed parallel to the hindbrain adjacent to, but a short distance lateral to rhombomeres 3–5 (C). At 12 hpf (5–6 somites), the patches take on an arced shape with a pronounced sox3-negative domain nearest the CNS (D). At later stages the single domain appears to extend in the rostrocaudal axis and break into a series of smaller patches (E) that are again clearly seen to be ectodermal in sections (more medial expression of sox3 also seen in the endoderm of the pharyngeal pouches) (F). Comparison of single in situ hybridization for ngn1 (G, H), neuroD (I, J) and sox3 (K, L) at 24 hpf shows that these regions the regions of neurogenesis are closely associated with the ectodermal patches of sox3 expression. This is most evident from sections taken at equivalent positions just rostral to the otic vesicle (H, J, L). Double in situ hybridization with the early marker of neurogenesis, ngn1 (red) at 24 hpf (M) and 36 hpf (N), verified that these sox3 domains (blue) are indeed the regions from which neurons later arise (M,N) as shown in the transverse section, inset in (M). (M□) and (N□) represent enlarged views of boxed region in (M) and (N) respectively. ad, anterodorsal lateral line; av, anteroventral lateral line; f, facial; m/v, middle lateral line/vagal; o/g, octaval/statoacoustic/glossopharyngeal; ov, otic vesicle. EXPRESSION / LABELING:
|
|
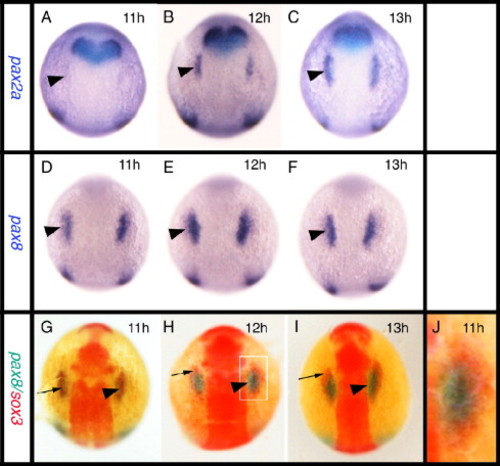
Relationship between the expression of sox3 and the otic markers pax2a and pax8. All panels viewed dorsally at the level of the hindbrain with rostral to the top. In situ hybridization for pax2a (A–C) or pax8 (D–F) expression. (G–J) Double in situ hybridization showing pax8 expression (blue) and sox3 expression (red). pax8 is expressed in the prospective otic placodes (arrowheads) first appearing as discrete domains between 9 and 11 hpf (D) when pax2a is barely detectable in the prospective otic region (arrowhead in A). Double in situ hybridization shows that the initial domain of pax8 expression (arrowhead) is largely coincident with the first expression of sox3 (arrow) in this region. By 12 hpf, however, when pax2a is strongly expressed (B, C), the two domains are almost mutually exclusive with pax8 (and pax2a, see also Fig. 5A) occupying a more medial domain and sox3 an arc of expression surrounding the pax8 expressing ectoderm (G–I). (I) Enlarged view of boxed domain in panel G. EXPRESSION / LABELING:
|
|
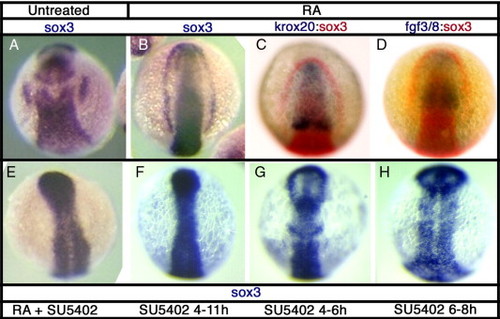
sox3 expression is affected by disruption of Fgf signaling. In situ hybridization for sox3 expression at 11 hpf in DMSO treated control embryos (A) or following treatments to alter Fgf signaling. Treatment with retinoic acid (RA) from 6–7 hpf causes expansion of the hindbrain, as shown by rostral displacement of the rhombomere 3 band of krox20 expression (C) and consequently expression of fgf3/8 throughout most of the rostral CNS (D). This treatment results in a striking expansion of sox3 expression, in the most severe cases resulting in a complete arc of expression around the anterior of the embryo (B). Expression of sox3 outside of the CNS, even in the presence of RA, is completely blocked when Fgf signaling is inhibited using the small molecule inhibitor, SU5402 (E–H). SU5402 causes a loss of placodal sox3 expression when treated from 4–11 hpf (F), but also after shorter treatments at 4–6 hpf (G) and 6–8 hpf (H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
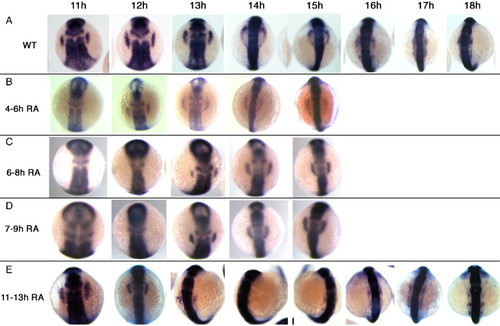
Fgf signaling is continuously required for maintenance of placodal sox3 expression. Embryos were treated with SU5402 to inhibit Fgf signaling for various periods of embryonic development, fixed at a range of time points and sox3 expression analysed by in situ hybridization. (A) sox3 expression in DMSO treated controls. (B) Inhibition of Fgf signaling from 4–6 hpf. Placodal expression of sox3 is delayed, only appearing at 12 hpf after which time expression is similar to wild type. Inhibition of Fgf signaling from 6–8 hpf (C) or 7–9 hpf (D) further delayed placodal expression of sox3, appearing at 13 hpf after which time expression is similar to wild type. (E) Inhibition of Fgf signaling after placodal sox3 expression was initiated, from 11–13 hpf. Placodal expression of sox3 is lost between 14 and 15 hpf, after which time it reappears in a pattern similar to wild-type. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Loss of both Fgf3 and Fgf8 is needed to inhibit all placodal sox3 expression. (A–F) Wild type or ace/fgf8 mutant embryos were analysed by double in situ hybridization at 12 hpf for sox3 (red) and pax2a (blue) expression with or without injection of a morpholino targeting expression of Fgf3 (or its mismatch control). Right hand of each panel shows enlarged view of left side placode. Injection of either wild type or ace mutants with the fgf3 mismatch control had no effect on either sox3 or pax2a expression (C, D). Injection of wild type embryos with the fgf3 morpholino resulted in a reduced domain of expression of both pax2a and sox3 expression (E) as compared to untreated wild type embryos (A). The ace mutant embryos did not appear to differ from wild type embryos in their sox3 or pax2a expression (B). However, when ace mutant embryos were injected with the fgf3 morpholino, complete loss of sox3 and pax2a expression was seen (F). (G–L) In situ for either pax2a (G–I) or sox3 (J–L) expression in wild type embryos injected with morpholinos that targeted either fgf3 (G,J), fgf8 (H,K) or both fgf3 and fgf8 together (I, L). As seen with injection of ace mutants with the fgf3 morpholino (F), only loss of both fgfs resulted in complete loss of pax2a or sox3 expression in the prospective placodes (I, L). EXPRESSION / LABELING:
PHENOTYPE:
|
|
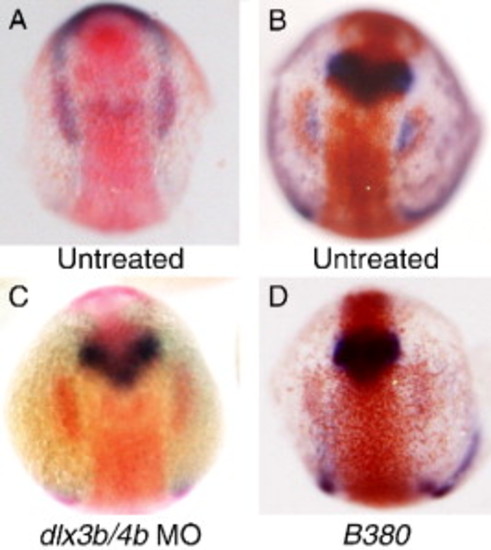
Relationship between dlx3b and sox3 expression. (A) Double in situ hybridization illustrating the appearance of placodal sox3 expression (red) at the caudal end of the pre-placodal domain of dlx3b expression (blue) at the 3-somite stage of development (11 hpf). (B–D) Double in situ hybridization for sox3 expression (red) and pax2a expression (blue) at 12 hpf. In untreated embryos expression of pax2a occupies a domain adjacent to, and medial to sox3 expression (B). Injection of embryos with morpholinos directed against both dlx3b and dlx4b results in loss of pax2a expression in the otic region, but has no effect on sox3 expression (C). Embryos homozygous for the B380 mutation show an identical loss of pax2a expression and normal sox3 expression (D). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Relationship between foxi1 and sox3 expression. All panels viewed dorsally at the level of the hindbrain with rostral to the top, except (A, B, E, G, H, K, M) which are viewed laterally. (A, B, G, H) dorsal to the right, (E, K, M) rostral to left. (A–F) Double in situ hybridization for foxi1 (blue) expression illustrating its changing expression in relation to krox20 (red) which marks rhombomeres 3 and 5 in the hindbrain as a positional point of reference. foxi1 expression develops from a single large domain either side of the hindbrain (A), gradually elongating and finally separating into almost distinct patches but always extending from a position slightly rostral to a position slightly caudal to rhombomeres 2–5 (B–F). However, from early somite stages (B) onwards, the level of expression decreases in the more caudal and lateral regions with strong expression maintained only in the rostral-most region (this is particularly evident in panels (I, J)). (G–L) Double in situ hybridization for sox3 (red) and foxi1 (green). (M, N) Single in situ hybridization for sox3 (blue). foxi1 expression outside of the CNS precedes placodal sox3 expression which only appears at tailbud/early somite stages (G–I). Prior to this stage, foxi1 exists as a shrinking domain either side of the hindbrain that does not directly abut the CNS expression of sox3 (G, H). As sox3 expression appears within the larger foxi1 domain of expression, there is a concomitant down regulation of foxi1 in the region of overlap (G, H). This is less apparent in (C, D) since in situ hybridization for foxi1 has been overdeveloped to reveal its full domain of expression. At later stages (K–N) the domains of sox3 and foxi1 remain overlapping with the same rostrocaudal pattern, although the sox3 expression lies more medially and the foxi1 extends more laterally. Note that at 24 hpf expression of sox3 in the underlying endoderm is seen as a particularly dark stain (arrows) below the more diffuse patches of ectodermal expression (outlined in panel N) when viewed dosally (L, N). (O–R) Effects of foxi1 morpholino. In situ hybridization at 12 hpf shows placodal expression of pax2a (O) and sox3 (Q) in wildtype embryos (arrowheads), is lost in embryos injected with the foxi1 morpholino, (P) and (R) respectively. EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Developmental Biology, 303(2), Sun, S.K., Dee, C.T., Tripathi, V.B., Rengifo, A., Hirst, C.S., and Scotting, P.J., Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent, 675-686, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.