- Title
-
The Extracellular Domain of Smoothened Regulates Ciliary Localization and Is Required for High-Level Hh Signaling
- Authors
- Aanstad, P., Santos, N., Corbit, K.C., Scherz, P.J., Trinh, L.A., Salvenmoser, W., Huisken, J., Reiter, J.F., and Stainier, D.Y.
- Source
- Full text @ Curr. Biol.
|
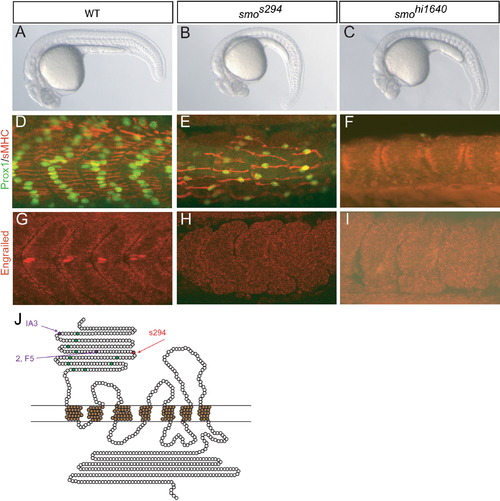
s294 Is a Hypomorphic Allele of smo with a C125Y Substitution in the Extracellular Domain (A–C) Comparison of wild-type (WT) (A), smos294 mutant (B), and smohi1640 mutant (C) embryos at 24 hr postfertilization (hpf) shows that s294 mutants have phenotypes similar to, although weaker than, the smo null allele hi1640. (D–F) Immunostaining for Prox1 (green) and slow myosin heavy chain (sMHC) (red) assesses medium- to low-level Hedgehog (Hh) signaling in WT (D), smos294 mutant (E), and smohi1640 mutant (F) embryos at 24 hpf. (G–I) Immunostaining for Engrailed (red) assesses high-level Hh signaling in WT (G), smos294 mutant (H), and smohi1640 mutant (I) embryos at 24 hpf. (J) Graphic representation of Smoothened (Smo). Each amino acid is represented by a circle; conserved cysteine residues in the extracellular domain (ECD) are shown in green. The s294 mutation is shown in red; similar Drosophila cysteine substitution alleles are shown in purple. |
|
The Extracellular Domain of Smo Is Required for High-Level Signaling (A–L) Purmorphamine treatment of WT and smos294 mutant embryos. DMSO-treated WT (A, E, and I), DMSO-treated smos294 mutant (C, G, and K), purmorphamine-treated WT (B, F, and J), and purmorphamine-treated smos294 mutant (D, H, and L) embryos at 24 hpf are shown. Purmorphamine treatment did not significantly affect morphology (B) and caused only a modest increase in Prox1 (green) and sMHC staining (red) (F) but strongly induced ectopic Engrailed (Eng)-positive muscle pioneer cells (MPs) in WT embryos (J). Purmorphamine treatment of smos294 mutants resulted in significant rescue of the morphological phenotype (D) and restored Prox1 (green) and sMHC (red) expression (H), but not Eng expression (L). (M–T) Injection of SmoΔCRD mRNA in WT and smohi1640 mutant embryos. Prox1 (green) and sMHC (red) staining (M–P) and Eng staining (Q–T) are shown. Injection of 250 pg SmoΔCRD mRNA caused a complete rescue of Prox1 and sMHC expression in smohi1640 mutants (P) compared to uninjected mutants (O) and a slight increase in Prox1 and sMHC expression in WT (N) compared to control (M). In contrast, injection of SmoΔCRD mRNA had no effect on Eng expression in WT (R) compared to control (Q) and did not rescue Eng expression in smohi1640 mutants (T). For quantification, see Tables S1 and S2. |
|
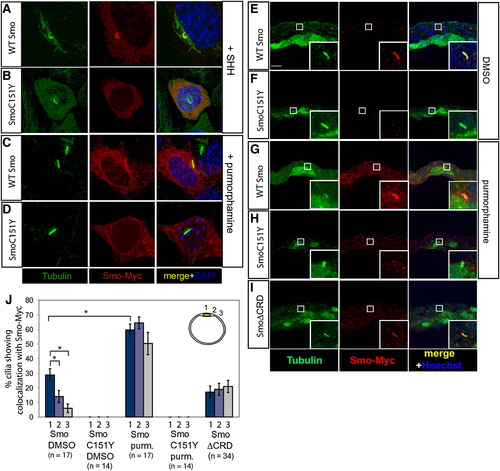
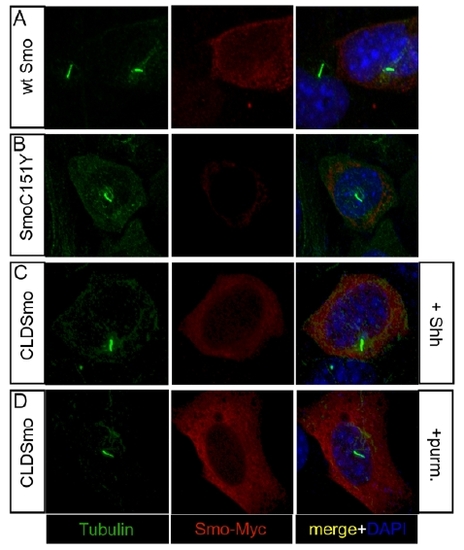
SmoC151Y Does Not Localize to the Cilium Expression of the ciliary marker acetylated tubulin (green) and either Myc-tagged WT Smo or Myc-tagged SmoC151Y (red) in NIH 3T3 cells (A–D) and zebrafish embryos at 10 hpf (E–J). Nuclei of NIH 3T3 cells were visualized with DAPI (blue). (A and B) In NIH 3T3 cells, WT Smo localized to the cilium in response to Hh (A), whereas SmoC151Y did not (B). (C and D) Treatment with the Smo agonist purmorphamine induced ciliary localization of WT Smo (C) but did not induce detectable ciliary localization of SmoC151Y (D). (E–I) Myc-tagged Smo mRNA was injected into Tg(-1.8gsc:GFP)ml1 zebrafish embryos, which express GFP in the dorsal midline. WT Smo localized to the cilia of cells surrounding the dorsal midline in embryos treated with DMSO (E), whereas SmoC151Y was not detected on the cilium (F). Purmorphamine treatment increased the ciliary localization of Smo (G) but did not induce the ciliary localization of SmoC151Y (H). In contrast, SmoΔCRD localized to the cilia in untreated embryos (I). Scale bar in (E) represents 10 μm. (J) The ciliary localization of Smo was quantified in locations away from the dorsal midline in sections (upper right), and the mean percentages of cilia exhibiting colocalization with the Myc-tagged Smo constructs are shown. Error bars indicate SEM. Total numbers of sections counted are shown below. |
|
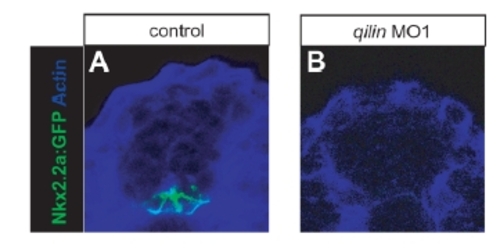
Inhibition of Ciliogenesis Causes Loss of Hh Target Gene Expression in Zebrafish Embryos (A–E) Expression of the ciliary marker acetylated tubulin (green) and the basal body marker γ-tubulin (red) in Kupffer′s vesicles of 16 hpf zebrafish embryos. Injection of dnKif3b mRNA (B), qilin morpholino 1 (MO1) (C), or qilin MO2 (D) caused a reduction of KV cilia, which were restored in embryos coinjected with qilin MO2 and qilin mRNA (E). Scale bar in (A) represents 10 μm. (F–O) In situ hybridization of 10 hpf control and injected embryos. (F–M) In WT embryos, ptc1 (F) and myod (K) are expressed in two stripes flanking the dorsal midline. Injection of dnKif3b (G), qilin MO1 (H), or qilin MO2 (I and L) caused a reduction or loss of ptc1 and myod expression, which could be restored by coinjection of qilin MO2 with qilin mRNA (J and M). (N and O) sonic hedgehog (shh) expression in qilin MO2-injected embryos (O) was comparable to controls (N), with a broadened expression reflecting convergence-extension defects. For quantification of these experiments, see Figure S10. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Purmorphamine stabilizes, but cannot induce full activity of SmoC151Y. |
|
SmoC151Y fails to localize to the cilium. |
|
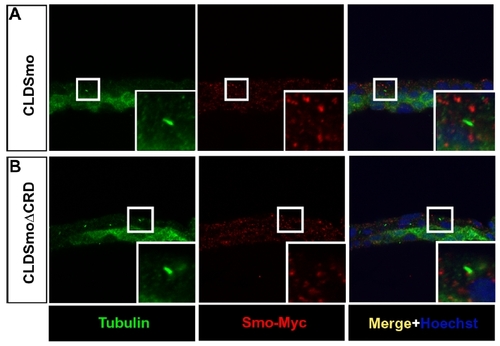
CLDSmo and CLDSmoΔCRD do not localize to cilia in zebrafish embryos. |
|
Areas of scanning. |
|
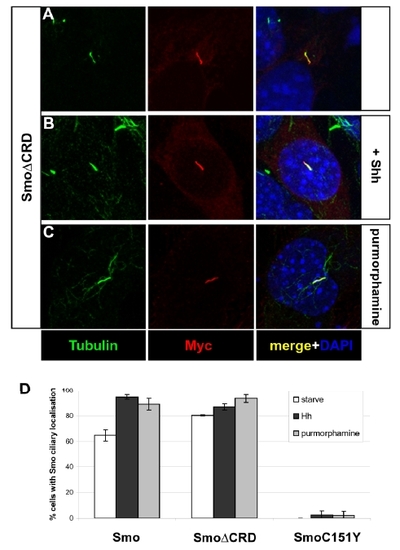
The ciliary localization of SmoΔCRD is independent of Hh pathway activation. |
|
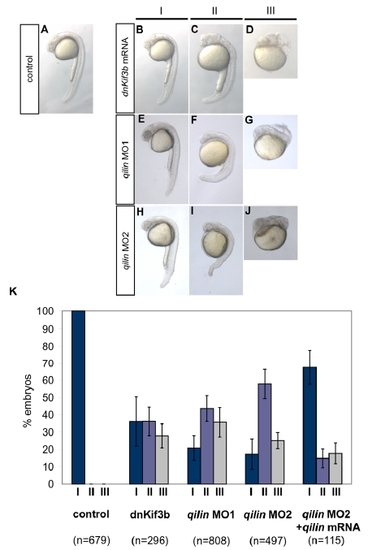
Morphological phenotypes in cilia knockdown embryos. |
|
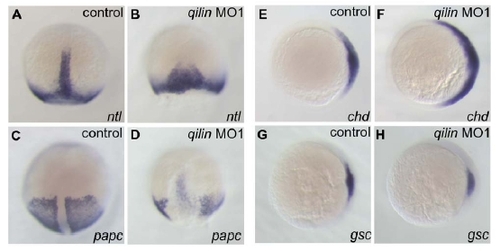
Convergence-extension and dorsal specification defects in cilia knock-down embryos |
|
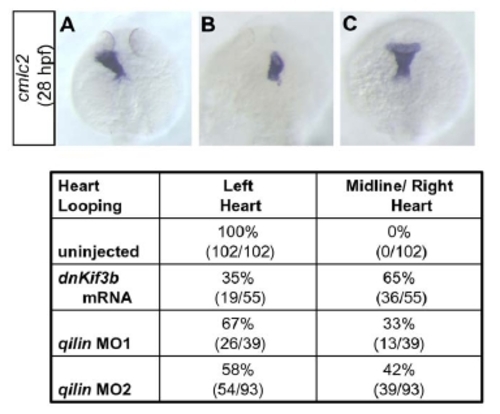
Laterality defects in cilia knock-down embryos. |
|
Variable loss of Hh target gene expression in later stage qilin morphant embryos. |