- Title
-
Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors
- Authors
- Hans, S., Liu, D., and Westerfield, M.
- Source
- Full text @ Development
|
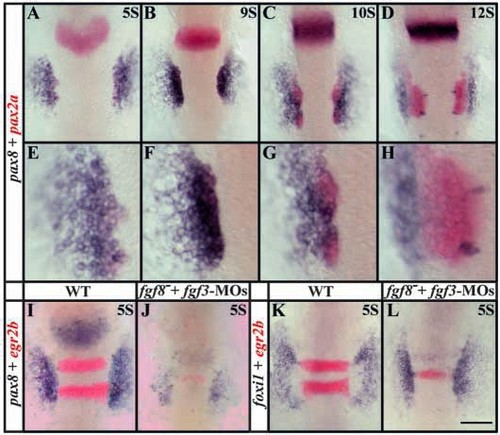
pax8 expression partially overlaps with pax2a in the preotic region and, like pax2a, depends upon Fgf signaling. Expression domains of pax8, (blue) and pax2a (red) coincide in the preotic region (A-C) but diverge after formation of the placode (D). At five-somite (A,E) to nine-somite (B,F) stages, the pax8 domain encompasses pax2a but also extends further laterally. By the 10- somite stage, when the otic placode is forming, pax8 expression diminishes in the placode (C,G) and is completely absent from the placode by the 12-somite stage (D,H) but is obvious just lateral to the placode. The bilateral pairs of medial blue spots in D,H are hindbrain neurons that express pax8. (E-H) High-magnification views of embryos shown in A-D. (I,J) Fgf3 and Fgf8 depletion leads to a severe reduction of pax8 expression at the five-somite stage but not to complete loss. In Fgf3- and Fgf8-depleted embryos, only weak expression can be detected (J) compared with uninjected wild-type embryos (I). Expression of egr2b (red) in the hindbrain (I) is also reduced when Fgf signals are lost (J) (Maves et al., 2002). (K,L) foxi1 is not dependent on the Fgf3 and Fgf8 signal. In wildtype embryos at the five-somite stage, foxi1 is expressed in two patches lateral to the neural plate (K) and depletion of Fgf3 and Fgf8 has no obvious effect on foxi1 expression (L). Dorsal views, anterior towards the top. Scale bar: 120 μm for A-D,I-L; 40 μm for E-H. EXPRESSION / LABELING:
|
|
Pax2a and Pax8 have overlapping functions in ear development. (A) Schematic map showing the genomic structure of pax8 (after Pfeffer et al., 1998), the protein domain structure and the positions of the splice-blocking morpholinos. Black arrows indicate MO E5/I5 and E9/I9, the most efficacious combination used in this study. The white arrows E2/I2, E3/I3 and E4/I4 show positions of the other three spliceblocking MOs that yielded only weak or no phenotype. The sequence of the first exon (?) is possibly incomplete. The efficacy of E5/I5 and E9/I9 pax8-MOs combination is visualized by in situ hybridization (B,C). (B) In wild-type embryos at the one-somite stage, pax8 transcripts in the preotic region are localized primarily in the cytoplasm leaving the nuclei relatively clear. (C) In E5/I5 and E9/I9 pax8-MOs injected embryos, pax8 transcripts are localized mostly in nuclei leaving the cytoplasm free of signal. (D-S) Knockdown of Pax8 in pax2a– mutants has a severe otic phenotype. Wild-type embryos (D,H,L,P), pax2a– mutants (E,I,M,Q) and pax8-MO-injected wild-type embryos (F,J,N,R) are similar, but, in contrast to pax2a– mutants, are depleted of Pax8 (G,K,O,S). The otic vesicle is absent in pax8-MO-injected pax2a– mutant embryos and cannot be detected at 22 h (G) or 50 h (K). However, Dlx3b (L-O) and cldna (P-S) are present in pax2a– mutants depleted of Pax8. (D-O) Side views, anterior towards the left, dorsal towards the top; (B,C,P-S) dorsal views, anterior towards the top. Scale bar: 30 μm for B,C; 200 μm for D-G; 75 μm for H-K; 60 μm for L-O; 100 μm for P-S. EXPRESSION / LABELING:
|
|
Pax2a and Pax8 are required for maintenance of otic cell fates. Pax2a and Pax8 are required together for preotic expression of sox9a (A-D), sox9b (E-H) and pax2a (M-P) but not of dlx3b (I-L). Cells of the presumptive otic placode express sox9a in wild-type embryos at the three-somite stage (A) at higher levels than in pax2a– mutants after pax8-MOs injection (B). At the 12-somite stage, sox9a is expressed throughout the otic placode in wild-type embryos (C) but no otic sox9a expression can be detected in pax2a– mutants depleted of Pax8 (D). (E-H) The sox9a duplicate, sox9b, shows similar behavior. In wild type at the five-somite stage, sox9b is expressed in the preotic region and neural crest (E). The neural crest expression is unaffected in pax2a– mutants injected with pax8-MOs, but expression in the preotic domain is reduced (F). At the 12-somite stage, sox9b is expressed strongly in the otic placode in wild-type embryos (G) but is absent in pax2a– mutants injected with pax8-MOs (H). dlx3b expression is strong in cells of the future otic placode in wild-type embryos (I) at the five-somite stage and this domain is smaller but still recognizable in pax2a– mutants after pax8-MOs injection (J). At the 12-somite stage, dlx3b is expressed throughout the otic placode in wild-type embryos (K) but in pax2a– mutants with a knockdown of Pax8, only a few residual cells express dlx3b (L). pax2a expression is strong in the otic placode of wild-type embryos at the 12-somite stage (M) but expression is severely reduced in pax2a– mutants after pax8-MOs injection (N). At 22 h, when the otic vesicle has formed in wild-type embryos, pax2a expression is restricted to the ventromedial region (O) but is completely absent in pax2a– mutants depleted of Pax8 (P). Expression of egr2b (red) in rhombomeres 3 and 5 is unchanged in pax2a– mutants after pax8- MOs injection (N) in comparison with uninjected wild-type embryos (M). Dorsal views, anterior towards the top. Scale bar: 120 μm. EXPRESSION / LABELING:
|
|
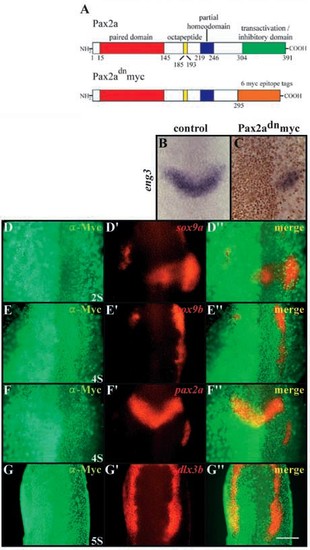
Pax2a and Pax8 are required for induction of otic cell fates. (A) Schematic map of protein domain structure of Pax2a and the dominant-negative variant, dnPax2a-myc. In dnPax2a-myc, the 295 N-terminal amino acids are fused in frame with six Myc-epitopes. The numbers indicate amino acids of the domains (Lun and Brand, 1998). (B,C) dnPax2a-myc acts in a dominant-negative fashion. At the three-somite stage, eng3 (blue) is expressed in the isthmic region (B), whereas in wild-type embryos injected with dnpax2a-myc, the side expressing the transgene shows reduced or no eng3 (blue) expression (C). The distribution of the Myc-epitope (brown) indicates the localization of the Pax2a variant in nuclei. Expression of dominant-negative Pax2a leads to downregulation of sox9a (D,D′,D′′), sox9b (E,E′,E′′) and pax2a (F,F′,F′′) but has only slight effects on dlx3b (G-G′). (D-G) Fluorescence images of a-Myc antibody labeling; (D′-G′) fluorescence images of mRNA in situ hybridization probes; (D′′-G′′) merged fluorescence images. (B-G′′) Dorsal views, anterior towards the top. Scale bar: 120 μm. EXPRESSION / LABELING:
|
|
Pax8 mediates the early Fgf-dependent induction of pax2a expression. In fgf8– mutant embryos at the five-somite stage, the preotic pax2a expression is reduced in size (B) compared with wildtype embryos of the same age (A). fgf8– mutant embryos after Pax8 depletion show an even further reduction of preotic pax2a expression (C). The defects in Pax2a expression are later manifested in a slightly reduced ear in fgf8– mutant embryos (E) or more severely reduced ear in fgf8– embryos depleted of Pax8 (F) in comparison with wildtype embryos at 50 h (D). (A-C) Dorsal views, anterior towards the top; (D-F) side views, anterior towards the left, dorsal towards the top. Scale bar: 120 μm for A-C; 180 μm for D-F. EXPRESSION / LABELING:
|
|
Foxi1 is required for patterning of otic precursors. The expression pattern of sox9a (A-D), sox9b (E-H), Dlx3b and Pax2 (I,J) are perturbed in foxi1- mutants. At the three-somite stage, sox9a expression is strong in cells of the future otic placode in wild-type embryos (A) but severely reduced in foxi1- mutants (B). At the 12- somite stage, sox9a is expressed throughout the otic placode in wild-type embryos (C) in contrast to foxi1- mutants of the same age that have only a few cells expressing sox9a (D). (E-H) Loss of foxi1 affects sox9b differently. At the five-somite stage, sox9b expression is reduced in the preotic region in foxi1- mutants (F) compared with wild-type embryos (E) but by the 12-somite stage, sox9b expression in foxi1- mutants has recovered (H), although not to wild-type levels (G). (I-J′) Dlx3b and Pax2 proteins coincide in the otic region. In wild-type embryos at the 12-somite stage, Dlx3b and Pax2 are co-expressed in cells throughout the otic placode (I-I′′). The overlapping pattern is also present in foxi1- mutants at this stage (J-J′′) showing that expression of one correlates with expression of the other. (A-H) Dorsal views, anterior towards the top; (I-J′) side views, anterior towards the left, dorsal towards the top. Scale bar: 120 μm for A-H; 50 μm for I,J. EXPRESSION / LABELING:
|
|
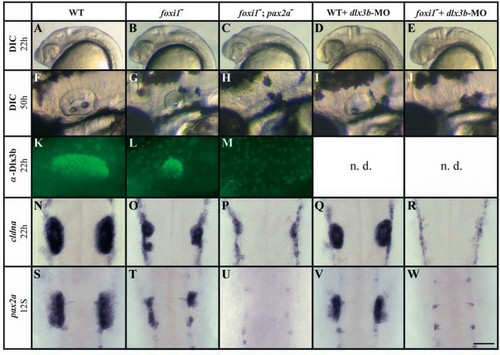
Foxi1 and Dlx3b mediate convergent pathways of otic development. In foxi1- mutants, otic tissue is reduced (B,G,L,O,T) compared with wild-type embryos (A,F,K,N,S) assessed both by morphology (A,B,F,G) and markers, including Dlx3b (K,L), cldna (N,O) and pax2a (S,T). foxi1-;pax2a– double mutants show no morphological sign of an otic vesicle (C,H) and no expression of Dlx3b (M) or pax2a (U), although some residual ‘otic’ cells can be detected with cldna (P). Injection of dlx3b-MO into wild-type embryos leads to reduction of overall ear size (D,I) that is preceded by reduced expression of cldna (Q) and pax2a (V). In foxi1- mutant embryos depleted of Dlx3b, all otic specification is absent as indicated by morphology (E,J) and transcription of cldna (R) and pax2a (W). (A-M) Side views, anterior towards the left, dorsal towards the top; (N-W) dorsal views, anterior towards the top. Scale bar: 200 μm for A-E; 75 μm for F-J; 60 μm for K-M; 100 μm for N-W. n.d., not done. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|