- Title
-
Junb controls lymphatic vascular development in zebrafish via miR-182
- Authors
- Kiesow, K., Bennewitz, K., Miranda, L.G., Stoll, S.J., Hartenstein, B., Angel, P., Kroll, J., Schorpp-Kistner, M.
- Source
- Full text @ Sci. Rep.
|
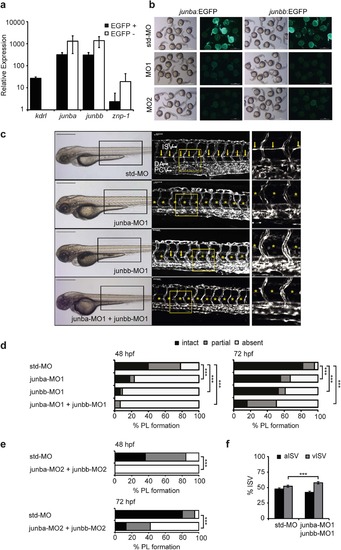
Junb knockdown in zebrafish causes failure in PL formation. (a) Expression analysis of cDNA isolated from EGFP + FAC-sorted cells from 24 hpf Tg(fli1:EGFP)y1 embryos. Junba and junbb are expressed in ECs at levels similar to the EC marker kdrl. The strong enrichment of kdrl in the GFP + and of the neuronal marker znp-1 in the GFP- fraction, respectively, proves a proper FAC-sorting. Error bars indicate Mean +/- SD (b) Wild type zebrafish embryos co-injected with 100 pg of a linearized junba:EGFP or junbb:EGFP reporter plasmid and 2 ng of the respective experimental or standard morpholino (std-MO) as indicated were assayed for reporter EGFP expression at 24 hpf. Embryos injected with either junb:EGFP-reporter construct and std-MO display strong EGFP expression, while either junba or junbb morpholino completely blocked the EGFP signal, respectively. Panel displays representative embryos from each injection group. (c) Left panel, bright field images of representative embryos at 72 hpf injected with the indicated morpholino: std-MO (4 ng, n = 104), junba-MO1 (2 ng, n = 115), junbb-MO1 (2 ng, n = 92), junba-MO1 + junbb-MO1 (2 ng + 2 ng, n = 108). Middle panel, confocal images of trunk vasculature in Tg(fli1:EGFP)y1 embryonic region marked by rectangle on the left. Right panel, enlarged view of trunk region marked by the yellow rectangle in the middle panel. DA, dorsal aorta; PCV, posterior cardinal vein; ISVs, intersegmental vessels; PL is marked with yellow arrows or if absent with asterisks. Panels are lateral view; dorsal is up, anterior to the left. Scale bars represent 500 Ám (black) and 50 Ám (white). (d,e) Percentage of PL defects in embryos shown in (c) at 48 and 72 hpf and for a non-overlapping second set of junb-specific translation-blocking MOs (MO-2). std-MO (n = 58), junba-MO2 + junbb-MO2 (n = 76). Sum of 16 hemisegments per embryo was categorized into groups with no, partial or complete absence of PL. ***P < 0.001, Mann-Whitney U-test. (e) Quantification of arterial and venous ISVs (aISV, vISV) given as % aISV and vISV in Tg(fli1:EGFP)y1 zebrafish embryos injected with indicated morpholinos at 72 hpf. Mean ▒ SD, n = 50-60 embryo/group. ***P < 0.001, Student?s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
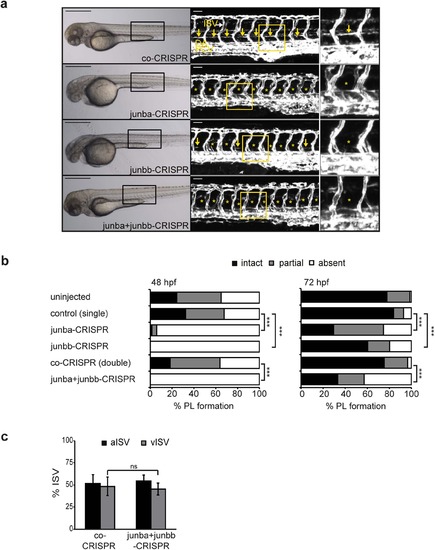
Junb knockout in zebrafish phenocopies the MO-mediated Junb knockdown PL phenotype. (a) Left panel: Bright field images of representative embryos at 72 hpf injected with the indicated CRISPR gRNA: co-CRISPR (400 pg), junba-CRISPR (250 pg), junbb-CRISPR (250 pg), junba-CRISPR + junbb-CRISPR (200 pg + 200 pg). Middle panel: Confocal images of trunk vasculature in Tg(fli1:EGFP)y1 embryonic region marked by rectangle on the left. Right panel, enlarged view of trunk region marked by the yellow rectangle in the middle panel. DA, dorsal aorta; PCV, posterior cardinal vein; ISVs, intersegmental vessels; PL marked with yellow arrows or if absent with asterisks. Panels are lateral view; dorsal is up, anterior to the left. Scale bars represent 500 Ám (black) and 50 Ám (white). (b) Percentage of PL defects in embryos (at 48 and 72 hpf) injected with CRISPR gRNAs as described in (a) against either junba (n = 68), junbb (n = 94) or in combination against both (n = 150) versus embryos injected with control CRISPR at corresponding doses of 250 pg (n = 99) or 400 pg (co-CRISPR, n = 105). Sum of 16 hemisegments per embryo was categorized into groups with no, partial or complete absence of PLs. ***P < 0.001, Mann-Whitney U-test. (c) Quantification of arterial and venous ISVs (aISV, vISV) given as % aISV and vISV in Tg(fli1:EGFP)y1 zebrafish embryos at 72 hpf injected with indicated CRISPR gRNAs. n = 40 embryo/group, mean ▒ SD. **P < 0.01, ns, not significant, Student?s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
miR-182 expression silencing recapitulates the Junb knockdown PL phenotype. (a) Sequences of the guide strand of targeted pre-miR-182 and of miR-182 (miR-182-MO) and 5-mismatch control (miR-182-5MM-MO) morpholinos used. Nucleotides of mature miR-182 and substituted nucleotides of miR-182-5MM-MO are marked in bold and red, respectively. (b) Dose-dependent knockdown of miR-182 as determined by Taqman miRNA assay in 48 hpf zebrafish embryos following injection of miR-182-MO, std-MO or miR-182-5MM-MO using the indicated MO concentrations. Data are normalized to U6 snRNA and expressed as mean ▒ SEM (n = 3). ***P < 0.001, **P < 0.01, Unpaired Student?s t-test for comparison to miR-182-5MM-MO. (c) Quantification of arterial and venous ISVs (aISV, vISV) given as % aISV and vISV in Tg(fli1:EGFP)y1 zebrafish embryos injected with indicated morpholinos at 72 hpf given as % aISV and % vISV. Mean ▒ SD. ns, not significant, Student?s t-test. (d) Left panel, representative bright field images of zebrafish embryos 72 hpf injected with miR-182-MO (12 ng, n = 151) compared to std-MO (12 ng, n = 107) and miR-182-5MM-MO (12 ng, n = 139). Middle panel, confocal images of trunk vasculature in respective embryonic region marked by rectangle on the left. Right panel, enlarged view of trunk region marked by the yellow rectangle in the middle panel. DA, dorsal aorta, PCV, posterior cardinal vein and ISV, intersegmental vessels. PL is marked with yellow arrows or if absent with asterisks. Panels are lateral view; dorsal is up, anterior to the left. Scale bars: 500 Ám (black) and 50 Ám (white). (e) Percentage of PL defects in embryos at 48 hpf and 72 hpf. ***P < 0.001, Mann-Whitney U-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
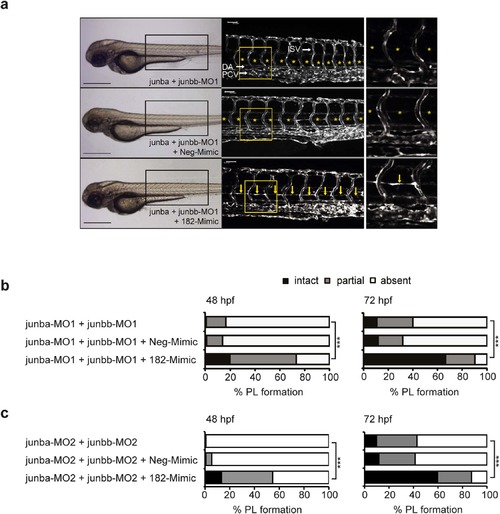
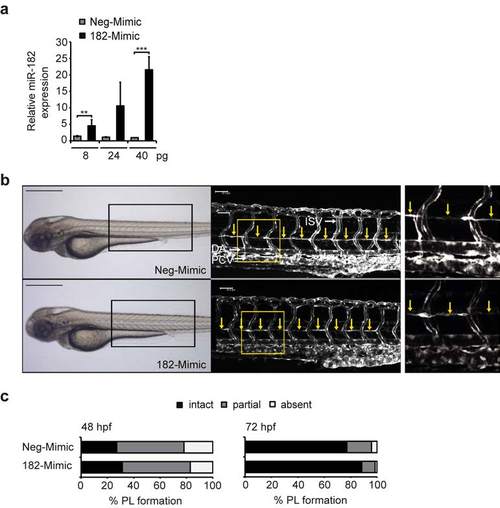
Ectopic miR-182 restores PL formation in Junb morphants. (a) Left panel, bright field images showing overall morphology of junba and junbb (2 + 2 ng) double morphants injected with or without Neg-Mimic (40 pg, n = 127) or 182-Mimic (40 pg, n = 109). Middle panel: Confocal images of indicated trunk region in Tg(fli1:EGFP)y1 embryos at 72 hpf. Right panel, enlarged view of trunk region marked by the yellow rectangle in the middle panel. DA, dorsal aorta, PCV, posterior cardinal vein and ISV, intersegmental vessels. PL is marked with yellow arrows or if absent with asterisks. Panels are lateral view; dorsal is up, anterior to the left. Scale bars: 500 Ám (black); 50 Ám (white). (b) Percentage of embryos injected with MOs and Mimics as indicated and (c) for a second set of junb-MOs: junba-MO2 + junbb-MO2 (2 ng + 2 ng, n = 112), with or without Neg-Mimic (40 pg, n = 145) or 182-Mimic (40 pg, n = 119). ***P < 0.001, Mann-Whitney U-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
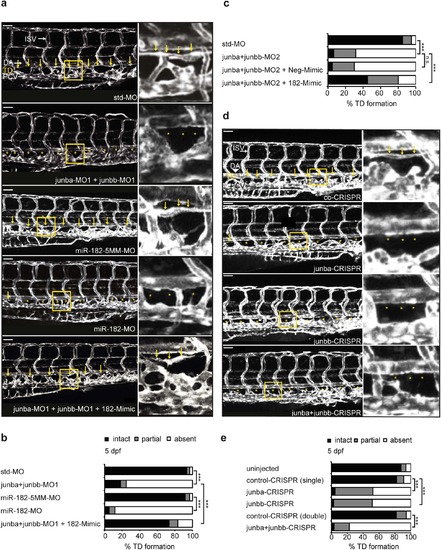
Failure in TD formation of Junb morphants is restored by ectopic miR-182. (a) Left panel, confocal images of trunk vasculature of representative larvae at 5 dpf injected with indicated morpholino or mimic: std-MO (4 ng, n = 62), junba-MO1 + junbb-MO1 (2 ng + 2 ng, n = 60), miR-182-5MM-MO (12 ng, n = 59), miR-182-MO (12 ng, n = 60), junba-MO1 + junbb-MO1 + 182-Mimic (2 ng + 2 ng + 40 pg, n = 32). Middle panel, confocal images of indicated trunk region. Right panel, enlarged view of trunk region marked by yellow rectangle in the middle panel. TD is located between dorsal aorta (DA) and posterior cardinal vein (PCV) and is marked with yellow arrows or if absent with asterisks. Panels are lateral view; dorsal is up, anterior to the left. White scale bar: 50 Ám. (b,c) Percentage of TD defects in larvae for the first (b) and the second set of junb-MOs (c). (d) Left panel, confocal images of trunk vasculature of representative larvae at 5 dpf CRISPR gRNA-injected larvae with indicated junb-CRISPR gRNAs or control CRISPR gRNA: junba-CRISPR (250 pg gRNA), junbb-CRISPR (250 pg gRNA), junba-CRISPR + junbb-CRISPR (200 pg + 200 pg gRNA) and Co-CRISPR (400 pg gRNA). Labelling and size bar as described in (a). (e) Percentage of TD defects in larvae at 5 dpf injected with CRISPR gRNAs either against junba, junbb or a combination of both versus larvae injected with control CRISPR gRNA. Sum of 10 somite segments per embryo was categorized into groups with presence, partial or complete absence of TD. ***P < 0.001, Mann-Whitney U-test. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
Expression of junb in zebrafish embryos and junb morpholino design and its validation. (a) Whole-mount in situ hybridization of 24 hpf embryos shows expression of junba and junbb mRNA in the eye an danal placode. Junbb mRNA can also be detected in the mesoderm and vasculature. Staining with sense control probes is negative. Bottom panels show positive controls for detection of junb at sites of injury (left panel) and in the eye (right panel). Size bars correspond 250 Ám in top panels, and 125 Ám in middle and bottom panels. (b) Total RNA was isolated from developing zebrafish embryos at 24, 48, 72, and 96 hpf and used to determine transcript levels of junba and junbb by semi-quantitative PCR. Actb1 served as loading control. (c) Upper panel: junb-MOs were designed to block translation of junba and junbb mRNAs due to the lack of exon-intron boundaries. Lower panel: gene sequences of regions targeted by junba-MO1/MO2 and junbb- MO1/MO2 around or upstream of start of translation. Start codon is underlined. (d) Schematic depiction of the 0.1 kb-long junb:EGFP fusion constructs created to determine the knockdown efficiency of the junb-MOs. A 0.1 kb segment of the 5′UTR/5′CDS of zebrafish junba or junbb carrying the binding sites for both experimental morpholinos (green line: MO1, MO2) was amplified from genomic zebrafish DNA, subcloned into pGEM-T, and further cloned inframe into pEGFP-N1. |
|
Ectopic administration of miR-182 does not cause vascular defects in zebrafish. (a) Overexpression of miR-182 was assessed by Taqman miRNA assay in 48 hpf zebrafish embryos injected with miR-182-mimic (182- Mimic) or negative control mimic (Neg-Mimic) as indicated mean ▒ SD (n=3-5). miR-182 expression level in 40 pg Neg-Mimic injected embryos was set to 1. *** P < 0.001, ** P < 0.01, Unpaired Student?s t-test. (B) Left panel, bright field images of embryos injected with 40 pg Neg-Mimic (n=129) or miR-182-Mimic (n=114). Middle panel, confocal images of indicated trunk region in Tg(fli1:EGFP)y1 embryos at 72 hpf. Right panel, enlarged view of trunk region marked by the yellow rectangle in the middle panel. DA, dorsal aorta, PCV, posterior cardinal vein, and ISV, intersegmental vessels. PL is marked with yellow arrows or, if absent, with asterisks. Panels are lateral views, dorsal is up, anterior to the left. Black scale bar: 500 Ám; white scale bar: 50 Ám. (c) Percentage of PL defects as described in Figure 5. |
|
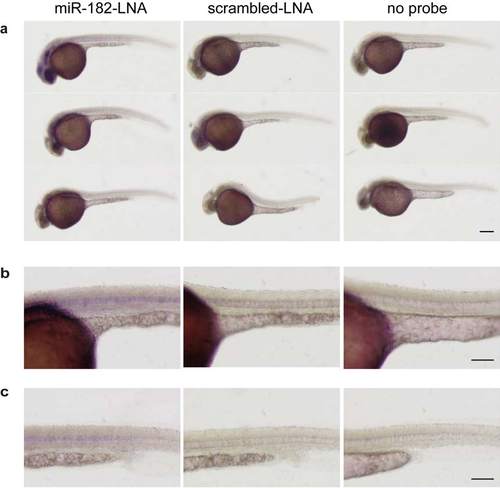
Analysis of miR-182 expression in 30 hpf embryos. (a-c) Whole-mount in situ hybridization of 30 hpf wild type embryos using miRCURY LNATM microRNA Detection Probes for miR-182 (miR-182-LNA, left panel) or a scrambled control (scrambled-LNA, middle panel). Right panel shows images of embryos incubated without LNA probe. (b) Higher magnification images of (a) showing the yolk sac and trunk region. (c) Higher magnification images showing soley the trunk region. miR-182 expression can be detected in eye lens, olfactory placode, optic tectum and notochord. Size bar corresponds to 250 Ám in (a) and 125 Ám in (b, c). EXPRESSION / LABELING:
|
|
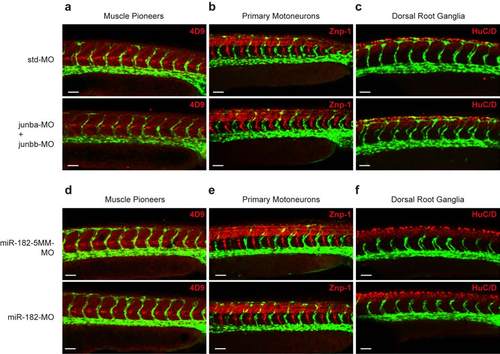
Non-endothelial structures supporting LC formation are not altered upon Junb or miR-182 knockdown. (a-f) Whole-mount immunostaining of co-MO or junba- and junbb-MO1 (a-c), miR-182-5MM-MO or miR-182-MO-injected (d-f) Tg(fli1:EGFP)y1 embryos at 30 hpf for (a, d) muscle pioneers (4D9, red), (b, e) primary motoneurons (Znp-1, red) and (c, f) dorsal root ganglia (HuC/D, red). Images show confocal projections of the trunk vasculature of representative embryos. For each condition we examined a minimum of 5 embryos. Panels are lateral view, dorsal is up, anterior to the left. Overall morphology of muscle pioneers, primary motoneurons and dorsal root ganglia showed no alterations in junba + junbb or miR-182 morphant embryos compared to their respective controls. White scale bar: 50 ÁM. |