- Title
-
PITX2 deficiency and associated human disease: insights from the zebrafish model
- Authors
- Hendee, K.E., Sorokina, E.A., Muheisen, S.S., Reis, L.M., Tyler, R.C., Markovic, V., Cuturilo, G., Link, B.A., Semina, E.V.
- Source
- Full text @ Hum. Mol. Genet.
|
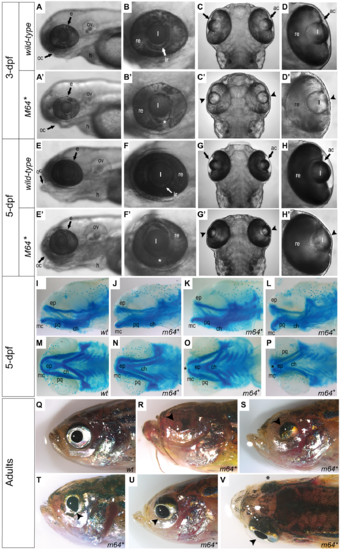
Gross characterization of the pitx2M64* mutant phenotype. (A–H’) Wild-type (A–H) and pitx2M64* mutant (A’–H’) embryos. At 3-dpf, prominent features include ventral iris coloboma (white asterisk in B’ versus B) and bilateral severely underdeveloped ocular anterior chambers (arrowheads in C’, D’ versus C, D). By 5-dpf, in addition to the persistence of the coloboma (white asterisks in F’ versus F) and underdeveloped anterior chambers (arrowheads in G’, H’ versus G, H), mutant lenses of homozygous pitx2M64* embryos appear elongated in the anterior/posterior plane (G’, H’ versus G–H). Craniofacial abnormalities also become apparent in mutants (E’ versus E). Views are lateral (A, B, A’, B’, E, F, E’, F’) and dorsal (C, D, C’, D’, G, H, G’, H’). (I–P) Alcian blue staining of 5-dpf wild-type (I, M) and pitx2M64* mutant (J–L, N–P) embryos. A range of mild to severe structural malformations were evident in the Meckel’s cartilage, mc, palatoquadrate, pq, (first pharyngeal arch) and ceratohyal, ch, (second pharyngeal arch) (I versus J–L, M versus N–P). Views are lateral (I–L) and ventral (M-P). (Q–V) Wild-type (Q) and pitx2M64* mutant (R–V) adult fish. Gross ocular phenotypic presentation included anophthalmia (R), microphthalmia (S), and enlarged/misshapen pupils with improper globe orientation (T, U) and could appear asymmetrically (as in V; arrowhead versus asterisk). Views are lateral (Q–U) and dorsal (V). ac, anterior chamber; e, eye; h, heart; ir, iris; l, lens; oc, oral cavity; ov, otic vesicle; re, retina; ep, ethmoid plate; mc, Meckel’s cartilage (P1); pq, palatoquadrate (P1); ch, ceratohyal (P2); hs, hyosympletic (P2). PHENOTYPE:
|
|
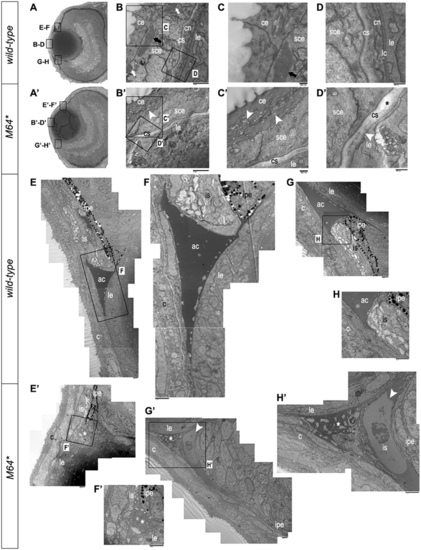
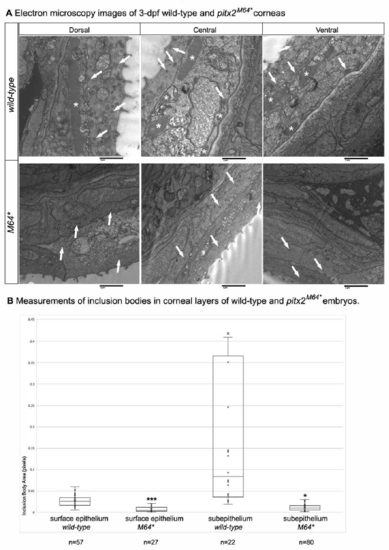
Electron microscopy of 3-dpf anterior chamber structures. (A, A’) Histological sections depicting orientation of wild-type (A) and pitx2M64* mutant (A’) eyes. (B–D, B’–D’) Wild-type (B–D) and pitx2M64* mutant (B’–D’) central corneas. Wild-type eyes display three layers of stratification: epithelium, stroma and endothelium (B). The corneal epithelium is delineated into surface and subepithelial cells distinguished by many dark-staining small inclusion bodies versus fewer larger inclusion bodies, respectively (arrows in B, C). The wild-type corneal stroma is of uniform thickness and on its basal side borders the corneal endothelium (D). In contrast, corneal defects in the mutants include an epithelium that contains smaller inclusion bodies in both layers and exhibits small vacuoles in the surface layer (arrowheads in B’, C’), a thicker and uneven stroma (asterisk in D’), and lack of a defined endothelial layer (arrowhead in D’). (E–H’) Wild-type (E–H) and pitx2M64* mutant (E’–H’) iridocorneal angles. Dorsal (E–F) and ventral (G–H) angles displayed early formation of the anterior chamber space as well as early differentiation of the iris stroma and iris pigmented epithelium. In mutants, both dorsal (E’, F’) and ventral (arrowheads in G’, H’) iridocorneal angles display notable under-differentiation of the iris stroma and iris pigmented epithelium, particularly ventrally; in addition, the anterior chamber space is marked by an excess of cellular material (asterisks in E’, H’). ce, corneal epithelium; cn, corneal endothelium; cs, corneal stroma; lc, lens capsule; le, lens epithelium; sce, corneal subepithelium; ac, anterior chamber; c, cornea; is, iris stroma; ipe, iris pigmented epithelium. PHENOTYPE:
|
|
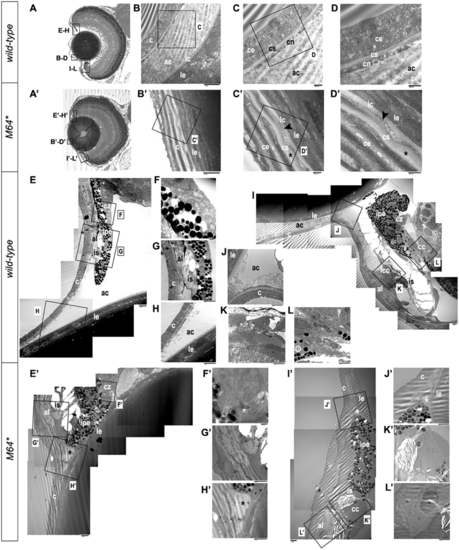
Electron microscopy of 14-dpf anterior chamber structures. Histological sections depicting orientation of wild-type (A) and pitx2M64* mutant (A’) eyes. Wild-type (B–D) and pitx2M64* mutant (B’–D’) central corneas. The wild-type cornea is clearly stratified into its three component layers: a three- to four-cell layer thick epithelium; a smooth, even-thickness stroma; and a uniform single layer endothelium (B–D). The corneal endothelium is distinctly separated from the lens capsule and epithelium by the anterior chamber space (B). In mutants, the anterior chamber space is absent, placing the mutant cornea directly up against the lens capsule (B’–D’). The innermost layer of subepithelium and stroma appear wavy and of fluctuating thickness (asterisks in C’, D’). The presumptive corneal endothelium, be it a monolayer endothelium or the compressed remnants of the anterior chamber space, is contoured between the stroma and lens capsule (arrowheads in C’, D’). Wild-type (E–L) and pitx2M64* mutant (E’–L’) iridocorneal angles. Wild-type dorsal (E–H) and ventral (I–L) angles display a distinct anterior chamber space with separation between lens and cornea and no angle occlusion (E, H–J); in addition, further differentiation of the iris stroma and the presumptive annular ligament is also demonstrated (E, G, I, K). Dorsally, the ciliary zone non-pigmented epithelial cells have begun to anteriorly differentiate from the retinal neuroepithelium (E, F). Ventrally, the formation of the iridocorneal canal and the ciliary canal, the two main branches of the specialized canalicular drainage network, can be observed (I, K, L). In contrast, the mutant shows a notable reduction in the anterior chamber space at both iridocorneal angles, with the iris pigmented epithelium remaining markedly in contact with the lens (asterisks in E’, H’–J’). The iris stroma and presumptive annular ligament appear delayed in their differentiation (E’, G’, I’, K’, L’). Dorsally, the non-pigmented ciliary epithelium does not appear to have begun differentiation (E’, F’). Ventrally, the only indication of specialization is the break in the iris pigmented epithelium marking the presumptive location of the ciliary canal (I’, K’); aside from this, the endothelial lining of the canal has failed to differentiate, and the iridocorneal drainage canal is undetectable (I’, K’, L’). ac, anterior chamber; c, cornea; ce, corneal epithelium; cn, corneal endothelium; cs, corneal stroma; lc, lens capsule; le, lens epithelium; al, annular ligament; cc, ciliary canal; cz, ciliary zone; icc, iridocorneal canal; ipe, iris pigmented epithelium; is, iris stroma. PHENOTYPE:
|
|
Histological analysis of adult pitx2M64* mutant phenotype. H&E transverse sections of wild-type (A, D) and pitx2M64* mutant (B, C, E, F) heads and corneas. Gross mutant phenotypes range from mild thickening of the corneal epithelium (C) to complete disorganization of the entire optic globe (B). The most prevalent phenotype includes the absence of both the anterior and posterior chambers as well as a severe reduction or a complete absence of the iris (B, C). In the cornea, the wild-type displays a typical stratified squamous epithelium, a uniformly thick stroma, and a single-layer endothelium (D). Mutant corneal epithelium is thicker, and a subset of cells takes on larger columnar morphology resembling that of epidermal stratified epithelium (black arrow in E). Mutant corneal stroma appears thinner, wavier, and sometimes interrupted (gray arrowhead in E). The analogous monolayer corneal endothelium region in the mutant is a noticeably thickened, disorganized layer of cells of unknown origin (black asterisk in E, F). Aberrant pigmentation is also typically observed in the mutant corneal epithelium and endothelium (black arrowheads in E, F), as well as cartilaginous layers (ca) in the anterior segment of mutant eyes (E, F). Some eyes that appeared opaque or hidden by gross analysis were found to be covered by skin and cartilage structures growing together over the eye globe (B, C, E, F). PAS staining of 1-mpf (G, H, M, N) and adult (I–L, O–Q) transverse sections. In wild-type adults 1-mpf and older, mucin-secreting goblet cells are present in the eye periphery (black arrows in G, H, J) but absent from the central corneal epithelium (G–I, K, L). In contrast, age-matched mutants develop goblet cells throughout the entire corneal epithelium (M–Q; black arrows in N and Q). Aberrant pigment deposits (black arrowheads in P, Q) and disorganized tissue next to the lens (asterisks in P, Q) are also evident. ac, anterior chamber; al, annular ligament; c, cornea; ca, cartilage; ir, iris; l, lens; re, retina; on, optic nerve; pc, posterior chamber. PHENOTYPE:
|
|
Immunohistochemical analysis of 1-mpf and adult pitx2M64* mutant phenotype. 1-mpf wild-type (A–D) and pitx2M64* mutant (E–L) sections stained with col1a1a (green), CKS (red) and DAPI (blue). D, H and L represent magnified images of the corresponding boxed regions in A, E and I. Wild-type corneal stroma staining reveals a continuous, uniform stromal layer with col1a1a (A, B, D) appearing both in the cornea (arrows in B and D) and around the eye (arrowhead in B) and CKS (A, C, D) being specific to the corneal stroma (arrows in C, D). In mutants, interruptions in or absence of col1a1a (E, F, H–J, L) and CKS (E, G–I, K, L) staining indicates disorganization of the corneal stroma (arrows in E–H, J–L). CKS also detects mucin-secreting goblet cells in the eye periphery and aberrantly approaching the corneal epithelium (I, K, L). Adult wild-type (M–O) and pitx2M64* mutant (Q–S) sections stained with col1a1a (green), CKS (red) and DAPI (blue). Wild-type adult corneal stroma continues to co-express col1a1a and CKS in a continuous layer (arrows in M and O) with CKS localized specifically to the cornea (arrows in M, N) and col1a1a also present around the eye (arrowheads in M, N). In mutant adults, col1a1a and CKS staining is absent from the corneal stroma region (Q). Instead, col1a1a stains ectopic cartilage structures in the endothelial region and ocular periphery (asterisks in Q, R,), whereas CKS stains mucin-secreting goblet cells in both the eye periphery and corneal epithelium (arrows in Q–S). Adult wild-type (P) and pitx2M64* mutant (T) sections stained with N-cadherin (green). In wild-type corneas, cdh2 detects both the corneal epithelium and the monolayer corneal endothelium (arrow in P). In mutants, while cdh2 staining was detectable but less robust in the epithelium, it did not appear in the endothelial region indicating the absence of a defined corneal endothelial layer (arrow in T). al, annular ligament; ce, corneal epithelium; cn, corneal endothelium; cs, corneal stroma; l, lens, re, retina. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
EXPRESSION / LABELING:
|
|
|