- Title
-
Waif1/5T4 Inhibits Wnt/β-Catenin Signaling and Activates Noncanonical Wnt Pathways by Modifying LRP6 Subcellular Localization
- Authors
- Kagermeier-Schenk, B., Wehner, D., Ozhan-Kizil, G., Yamamoto, H., Li, J., Kirchner, K., Hoffmann, C., Stern, P., Kikuchi, A., Schambony, A., and Weidinger, G.
- Source
- Full text @ Dev. Cell
|
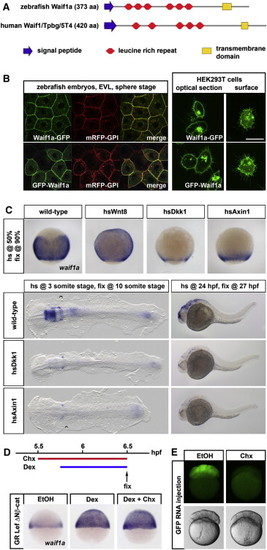
Zebrafish waif1a Is a Direct Universal β-Catenin Target Gene Encoding a Transmembrane Protein Localized to the Plasma Membrane(A) Domain structure of zebrafish Waif1a and human Waif1/5T4.(B) Localization of GFP-tagged zebrafish Waif1a in enveloping cells (EVL) of sphere stage zebrafish embryos and in HEK293T cells.(C) waif1a expression assessed by in situ hybridization in zebrafish embryos expressing Wnt8, Dkk1, or Axin1 (n [90%] = Wnt8, 13 of 13 embryos, Dkk1 18 of 18, Axin1 15 of 15; n [3 somites] = Dkk1 15 of 15, Axin1 18 of 20; n [24 hpf] = Dkk1 15 of 15, Axin1 14 of 14). (D) waif1a upregulation in embryos injected with GR Lef ΔNβ-cat RNA and treated with dexamethasone (Dex, 24 of 24 embryos) and in embryos pretreated with Cycloheximide (Chx, 22 of 22 embryos).(E) GFP expression in GFP RNA-injected embryos after 1 hr of EtOH or Chx treatment. See also Figure S1. EXPRESSION / LABELING:
|
|
Zebrafish waif1a Is a Direct Universal β-Catenin Target Gene Encoding a Transmembrane Protein Localized to the Plasma Membrane (A) Domain structure of zebrafish Waif1a and human Waif1/5T4. (B) Localization of GFP-tagged zebrafish Waif1a in enveloping cells (EVL) of sphere stage zebrafish embryos and in HEK293T cells. (C) waif1a expression assessed by in situ hybridization in zebrafish embryos expressing Wnt8, Dkk1, or Axin1 (n [90%] = Wnt8, 13 of 13 embryos, Dkk1 18 of 18, Axin1 15 of 15; n [3 somites] = Dkk1 15 of 15, Axin1 18 of 20; n [24 hpf] = Dkk1 15 of 15, Axin1 14 of 14). (D) waif1a upregulation in embryos injected with GR Lef ?Nβ-cat RNA and treated with dexamethasone (Dex, 24 of 24 embryos) and in embryos pretreated with Cycloheximide (Chx, 22 of 22 embryos). (E) GFP expression in GFP RNA-injected embryos after 1 hr of EtOH or Chx treatment. See also Figure S1. |
|
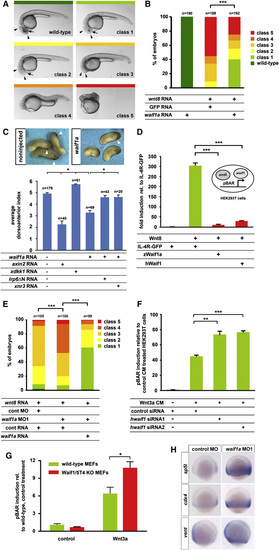
Waif1a Inhibits Wnt/β-Catenin Signaling (A) Classes of phenotypes induced by wnt8 overexpression: class 1, small eyes (arrow); class 2, no eyes (arrow); class 3, reduced forebrain and midbrain (arrowheads); class 4, loss of notochord indicative of enhancement of zygotic, organizer-restricting Wnt signaling; and class 5, hyperdorsalized, indicative of activation of maternal, organizer-inducing Wnt signaling. (B) Distribution of phenotypes in embryos injected with wnt8 (8 pg) plus waif1a RNA (60 pg) or equimolar amounts of GFP control RNA. ***p < 0.001, chi-square test. (C) Dorsoventral patterning phenotypes indicated by loss or reduction of cement glands (arrowheads) and quantified using the DAI of Xenopus embryos injected on the dorsal side with waif1a RNA (200 pg), axin2 (conductin, 500 pg), xdkk1 (100 pg), lrp6ΔN (60 pg), and xnr3 (200 pg). *p < 0.05, Student′s t test. (D) pBAR reporter activity (relative to IL-4R-GFP control) in HEK293T cells transfected with the indicated constructs. ***p < 0.001, Student′s t test. (E) Phenotypic classes as defined in (A) of embryos injected with 8 pg wnt8 RNA, 4 ng waif1a MO1, and 32 pg of MO-insensitive waif1a RNA or equimolar control reagents. ***p < 0.001, chi-square-test. (F) Waif1/5T4 knockdown in HEK293T cells enhances pBAR activation in response to Wnt3a conditioned media. **p < 0.01 and ***p < 0.001, Student′s t test. (G) Waif1/5T4 knockout MEFs show increased pBAR activation in response to purified Wnt3a (100 ng/μl). *p < 0.05, two-way ANOVA with Bonferroni′s post hoc test. (H) Whole-mount in situ hybridization reveals upregulation of sp5l (15 of 16 embryos), cdx4 (15 of 15), and vent (15 of 16) at the 90% epiboly stage in embryos injected with10 ng waif1a MO1. Note that staining reactions were stopped before the normal wild-type expression in control embryos became visible. See also Figure S2. EXPRESSION / LABELING:
|
|
Waif1a Inhibits Wnt/β-Catenin Signaling (A) Classes of phenotypes induced by wnt8 overexpression: class 1, small eyes (arrow); class 2, no eyes (arrow); class 3, reduced forebrain and midbrain (arrowheads); class 4, loss of notochord indicative of enhancement of zygotic, organizer-restricting Wnt signaling; and class 5, hyperdorsalized, indicative of activation of maternal, organizer-inducing Wnt signaling. (B) Distribution of phenotypes in embryos injected with wnt8 (8 pg) plus waif1a RNA (60 pg) or equimolar amounts of GFP control RNA. p < 0.001, chi-square test. (C) Dorsoventral patterning phenotypes indicated by loss or reduction of cement glands (arrowheads) and quantified using the DAI of Xenopus embryos injected on the dorsal side with waif1a RNA (200 pg), axin2 (conductin, 500 pg), xdkk1 (100 pg), lrp6ΔN (60 pg), and xnr3 (200 pg). p < 0.05, Student′s t test. (D) pBAR reporter activity (relative to IL-4R-GFP control) in HEK293T cells transfected with the indicated constructs. p < 0.001, Student′s t test. (E) Phenotypic classes as defined in (A) of embryos injected with 8 pg wnt8 RNA, 4 ng waif1a MO1, and 32 pg of MO-insensitive waif1a RNA or equimolar control reagents. p < 0.001, chi-square-test. (F) Waif1/5T4 knockdown in HEK293T cells enhances pBAR activation in response to Wnt3a conditioned media. p < 0.01 and p < 0.001, Student′s t test. (G) Waif1/5T4 knockout MEFs show increased pBAR activation in response to purified Wnt3a (100 ng/µl). p < 0.05, two-way ANOVA with Bonferroni′s post hoc test. (H) Whole-mount in situ hybridization reveals upregulation of sp5l (15 of 16 embryos), cdx4 (15 of 15), and vent (15 of 16) at the 90% epiboly stage in embryos injected with10 ng waif1a MO1. Note that staining reactions were stopped before the normal wild-type expression in control embryos became visible. See also Figure S2. EXPRESSION / LABELING:
PHENOTYPE:
|
|
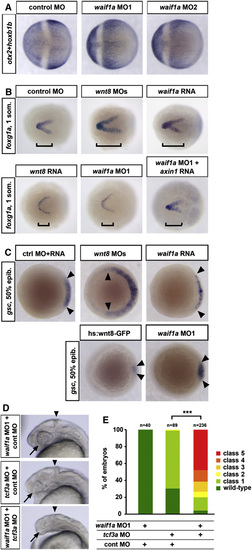
waif1a Inhibits wnt8 Function in Mesoderm and Neuroectoderm Patterning (A) Reduction of the forebrain marker otx2 and expansion of the posterior neural marker hoxb1b at the 100% epiboly stage in embryos injected with 10 ng waif1a MO1 or MO2 (23 of 28 embryos MO1, 21 of 24 MO2). (B) Changes in size of the telencephalic foxg1a expression domain at the one somite stage (bracket) in embryos injected with 2 ng each of wnt8.1 and wnt8.2 MOs (20 of 21 embryos), 160 pg waif1a RNA (36 of 36), 0.4 pg wnt8 RNA (22 of 26), and 4 ng of waif1a MO1 (16 of 25) without or with 2 pg axin1 RNA (17 of 23). (C) Changes in the size of the dorsal gsc expression domain at the 50% epiboly stage (arrowheads) in embryos injected with 2 ng each of wnt8.1 and wnt8.2 MOs (41 of 42 embryos), 150 pg waif1a RNA (35 of 44), and 10 ng waif1a MO1 (20 of 21 embryos), relative to embryos injected with equimolar amounts of luciferase control RNA plus control MO and in hsp70l:Wnt8-GFP transgenic embryos heat shocked at 30% epiboly (46 of 46 embryos). (D) Head regions with eyes (arrows) and midbrain-hindbrain boundary (arrowheads) indicated at 24 hpf of embryos injected with 10 ng waif1a MO1 and 0.2 ng standard control MO, or 0.2 ng tcf3a MO plus 10 ng control MO. (E) Quantification of the tcf3a and waif1a knockdown synergy. Embryos were classified using the criteria introduced in Figure 2A, ***p < 0.001, chi-square test. See also Figure S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
waif1a Inhibits wnt8 Function in Mesoderm and Neuroectoderm Patterning (A) Reduction of the forebrain marker otx2 and expansion of the posterior neural marker hoxb1b at the 100% epiboly stage in embryos injected with 10 ng waif1a MO1 or MO2 (23 of 28 embryos MO1, 21 of 24 MO2). (B) Changes in size of the telencephalic foxg1a expression domain at the one somite stage (bracket) in embryos injected with 2 ng each of wnt8.1 and wnt8.2 MOs (20 of 21 embryos), 160 pg waif1a RNA (36 of 36), 0.4 pg wnt8 RNA (22 of 26), and 4 ng of waif1a MO1 (16 of 25) without or with 2 pg axin1 RNA (17 of 23). (C) Changes in the size of the dorsal gsc expression domain at the 50% epiboly stage (arrowheads) in embryos injected with 2 ng each of wnt8.1 and wnt8.2 MOs (41 of 42 embryos), 150 pg waif1a RNA (35 of 44), and 10 ng waif1a MO1 (20 of 21 embryos), relative to embryos injected with equimolar amounts of luciferase control RNA plus control MO and in hsp70l:Wnt8-GFP transgenic embryos heat shocked at 30% epiboly (46 of 46 embryos). (D) Head regions with eyes (arrows) and midbrain-hindbrain boundary (arrowheads) indicated at 24 hpf of embryos injected with 10 ng waif1a MO1 and 0.2 ng standard control MO, or 0.2 ng tcf3a MO plus 10 ng control MO. (E) Quantification of the tcf3a and waif1a knockdown synergy. Embryos were classified using the criteria introduced in Figure 2A, p < 0.001, chi-square test. See also Figure S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
waif1a Interacts with LRP6 and Inhibits Wnt3a-Induced LRP6 Internalization without Affecting LRP6 Phosphorylation (A) Waif1a-GFP and Waif1/CD44/Waif1-GFP coimmunoprecipitate with LRP6-HA, but not LDLR-HA in HEK293T cells. (B) Waif1a-GFP does not block Wnt3a-induced phosphorylation of LRP6 at S1490 in HEK293T cells assayed at 8 hr poststimulation with Wnt3a CM, whereas Dkk1 overexpression does. (C?E) Subcellular localization of Flag-LRP6 and S1490 phosphorylated LRP6 in HEK293T cells treated with Wnt3a CM (C) and in cells transfected with Waif1a-GFP (D) or Waif1c-GFP (E). Scale bars represent 5 μm. (F) Quantification of Wnt3a-induced Flag-LRP6 internalization. membrane, clear localization to the cell surface, few puncta in cytosol; membrane + cytosol, localization of puncta to cell surface and puncta in cytosol; cytosol, no cell surface signal, more than 20 puncta in the cytosol. n = 110 Flag-LRP6, -Wnt3a; n = 105 Flag-LRP6, +Wnt3a; n = 107 Flag-LRP6+Waif1a-GFP, +Wnt3a; n = 114 Flag-LRP6+Waif1c-GFP, +Wnt3a. (G) Cell surface LRP6 detected by biotinylation and total LRP6 in control or Waif1a-Flag transfected cells treated with Wnt3a. Lower panel shows ratio of cell surface LRP6 (top) to total LRP6 (middle) with values at zero Wnt3a set to 100%. Means ± SEM from three independent experiments. See also Figure S6. |
|
waif1a Interacts with LRP6 and Inhibits Wnt3a-Induced LRP6 Internalization without Affecting LRP6 Phosphorylation (A) Waif1a-GFP and Waif1/CD44/Waif1-GFP coimmunoprecipitate with LRP6-HA, but not LDLR-HA in HEK293T cells. (B) Waif1a-GFP does not block Wnt3a-induced phosphorylation of LRP6 at S1490 in HEK293T cells assayed at 8 hr poststimulation with Wnt3a CM, whereas Dkk1 overexpression does. (C?E) Subcellular localization of Flag-LRP6 and S1490 phosphorylated LRP6 in HEK293T cells treated with Wnt3a CM (C) and in cells transfected with Waif1a-GFP (D) or Waif1c-GFP (E). Scale bars represent 5 µm. (F) Quantification of Wnt3a-induced Flag-LRP6 internalization. membrane, clear localization to the cell surface, few puncta in cytosol; membrane + cytosol, localization of puncta to cell surface and puncta in cytosol; cytosol, no cell surface signal, more than 20 puncta in the cytosol. n = 110 Flag-LRP6, -Wnt3a; n = 105 Flag-LRP6, +Wnt3a; n = 107 Flag-LRP6+Waif1a-GFP, +Wnt3a; n = 114 Flag-LRP6+Waif1c-GFP, +Wnt3a. (G) Cell surface LRP6 detected by biotinylation and total LRP6 in control or Waif1a-Flag transfected cells treated with Wnt3a. Lower panel shows ratio of cell surface LRP6 (top) to total LRP6 (middle) with values at zero Wnt3a set to 100%. Means ± SEM from three independent experiments. See also Figure S6. |
|
waif1a Enhances β-Catenin-Independent Wnt Signaling in Zebrafish Embryos (A) Classes of phenotypes induced in hsp70l:wnt5b-GFP transgenic zebrafish embryos heat shocked during gastrulation: class 1, shortening of the yolk extension (black line) by up to 1/3; class 2, up to 1/2; and class 3, more severe shortening plus other morphogenesis defects including cyclopia or open neural tube. (B) Phenotypic classes in waif1a RNA (75 pg) or RLuc (80 pg)-injected hsp70l:wnt5b-GFP embryos after weak induction (left, heat shock 39°C shield stage; ***p < 0.001, chi-square test) or strong induction (right, heat shock 40°C, 80% epiboly; *p < 0.05, chi-square test). (C and D) hsp70l:wnt5-GFP embryos injected with standard control (cont) MO (8 ng) plus RLuc control RNA (30 pg), waif1a MO1 plus waif1a MO2 (5 ng each) plus RLuc RNA, or waif1a MOs plus MO-insensitive waif1a RNA (32 pg), heat shocked at 50% epiboly, and photographed at 48 hpf. Body length (from the otic vesicle to the tip of the tail, arrows) is plotted in (D). ***p < 0.001, Student′s t test. not sign., not significant. See also Figure S7. |
|
waif1a Enhances β-Catenin-Independent Wnt Signaling in Zebrafish Embryos (A) Classes of phenotypes induced in hsp70l:wnt5b-GFP transgenic zebrafish embryos heat shocked during gastrulation: class 1, shortening of the yolk extension (black line) by up to 1/3; class 2, up to 1/2; and class 3, more severe shortening plus other morphogenesis defects including cyclopia or open neural tube. (B) Phenotypic classes in waif1a RNA (75 pg) or RLuc (80 pg)-injected hsp70l:wnt5b-GFP embryos after weak induction (left, heat shock 39°C shield stage; p < 0.001, chi-square test) or strong induction (right, heat shock 40°C, 80% epiboly; p < 0.05, chi-square test). (C and D) hsp70l:wnt5-GFP embryos injected with standard control (cont) MO (8 ng) plus RLuc control RNA (30 pg), waif1a MO1 plus waif1a MO2 (5 ng each) plus RLuc RNA, or waif1a MOs plus MO-insensitive waif1a RNA (32 pg), heat shocked at 50% epiboly, and photographed at 48 hpf. Body length (from the otic vesicle to the tip of the tail, arrows) is plotted in (D). p < 0.001, Student′s t test. not sign., not significant. See also Figure S7. |
|
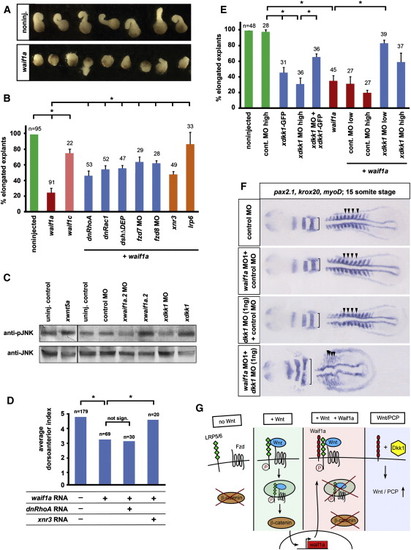
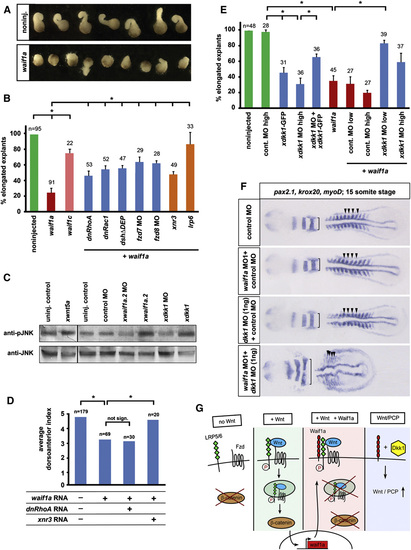
Waif1a Activates Noncanonical Wnt Signaling by a Dkk1-Dependent Mechanism (A) Overexpression of zebrafish waif1a RNA (200 pg) inhibits CE movements of Xenopus Keller explants. noninj., noninjected. (B) Fraction of elongated explants: waif1a, 200 pg; waif1c, 200 pg; dnRhoA, 50 pg; dnRac1, 50 pg; DshΔDEP, 500 pg; frizzled 7 MO, 1.6 pmol; frizzled 8 MO, 1.6 pmol; xnr3, 200 pg; lrp6, 60 pg. *p < 0.05, Student′s t test. The average of three independent experiments is shown. (C) Western blot of Xenopus embryo extracts at stage 10.5 blotted for endogenous phospho-JNK and total JNK. xwnt5a, 100 pg; xwaif1a.2, 50 pg; xdkk1, 100 pg; xwaif1a.2 MO, 1.6 pmol; xdkk1 MO, 1.6 pmol. (D) dnRhoA (50 pg) does not, but xnr3 (200 pg) can, fully rescue zebrafish waif1a (200 pg)-induced ventralization of Xenopus embryos quantified using the DAI. *p < 0.05, Student′s t test. (E) Fraction of elongated explants. dkk1 MO low, 0.8 pmol; high, 1.6 pmol. The average of three independent experiments is shown. *p < 0.05, Student′s t test. (F) CE defects in zebrafish embryos coinjected with waif1a MO1 and dkk1 MO (52 of 61 embryos) shown by compressed myoD-positive somites (arrowheads) and widened krox20 and pax2.1-positive neural expression domains (brackets) at the 15 somite stage. waif1a MO1, 2 ng; control MO, 2 ng; dkk1 MO, 1 ng. (G) Model of Waif1a function as modifier of Wnt signaling pathways. waif1a expression is directly regulated by β-catenin signaling during gastrulation and acts as a cell-autonomous feedback inhibitor of the pathway in Wnt-receiving cells. Waif1 interacts with LRP6 and interferes with Wnt-dependent internalization of LRP6, without affecting LRP/Frizzled interaction or LRP6 phosphorylation. See also Figure S8. PHENOTYPE:
|
|
Waif1a Activates Noncanonical Wnt Signaling by a Dkk1-Dependent Mechanism (A) Overexpression of zebrafish waif1a RNA (200 pg) inhibits CE movements of Xenopus Keller explants. noninj., noninjected. (B) Fraction of elongated explants: waif1a, 200 pg; waif1c, 200 pg; dnRhoA, 50 pg; dnRac1, 50 pg; DshΔDEP, 500 pg; frizzled 7 MO, 1.6 pmol; frizzled 8 MO, 1.6 pmol; xnr3, 200 pg; lrp6, 60 pg. p < 0.05, Student′s t test. The average of three independent experiments is shown. (C) Western blot of Xenopus embryo extracts at stage 10.5 blotted for endogenous phospho-JNK and total JNK. xwnt5a, 100 pg; xwaif1a.2, 50 pg; xdkk1, 100 pg; xwaif1a.2 MO, 1.6 pmol; xdkk1 MO, 1.6 pmol. (D) dnRhoA (50 pg) does not, but xnr3 (200 pg) can, fully rescue zebrafish waif1a (200 pg)-induced ventralization of Xenopus embryos quantified using the DAI. p < 0.05, Student′s t test. (E) Fraction of elongated explants. dkk1 MO low, 0.8 pmol; high, 1.6 pmol. The average of three independent experiments is shown. p < 0.05, Student′s t test. (F) CE defects in zebrafish embryos coinjected with waif1a MO1 and dkk1 MO (52 of 61 embryos) shown by compressed myoD-positive somites (arrowheads) and widened krox20 and pax2.1-positive neural expression domains (brackets) at the 15 somite stage. waif1a MO1, 2 ng; control MO, 2 ng; dkk1 MO, 1 ng. (G) Model of Waif1a function as modifier of Wnt signaling pathways. waif1a expression is directly regulated by β-catenin signaling during gastrulation and acts as a cell-autonomous feedback inhibitor of the pathway in Wnt-receiving cells. Waif1 interacts with LRP6 and interferes with Wnt-dependent internalization of LRP6, without affecting LRP/Frizzled interaction or LRP6 phosphorylation. See also Figure S8. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Waif phylogeny and expression during zebrafish and Xenopus embryogenesis and creation of a transgenic zebrafish line expressing Axin1-YFP after heatshock. Data characterizing a newly created hs:axin1YFP transgenic fish line are presented, as well as, the primary sequence conservation of waif family members, the phylogenetic relationship of zebrafish and mammalian Waif proteins, the expression pattern of zebrafish waif1a and Xenopus waif1a.2 in wildtype embryos and data showing that waif1a expression is regulated by wnt8 in zebrafish embryos. Waif phylogeny and expression during zebrafish and Xenopus embryogenesis and creation of a transgenic zebrafish line expressing Axin1-YFP after heat-shock, related to figure 1 (A-C) Waif1a is part of a family of proteins of which zebrafish contain four members and humans two. (A) Multiple sequence alignment of human and mouse Waif1 (Trophoblast glycoprotein / 5T4) with zebrafish Waif1a. Identical amino acids are highlighted in yellow, conservative exchanges are in grey. The predicted signal sequence, leucine rich repeat regions, the predicted transmembrane domain and presumptive N-glycosylation sites are indicated. (B) Percentage of identical and similar (second number in each field) amino acids between the zebrafish and human proteins are shown. (C) Phylogenetic relationship of human, mouse, and zebrafish Waif proteins. An unrooted phylogenetic tree was created using the Neighbor-joining method. Accession numbers of proteins used: h waif1= Human 5T4 NP_006661, m waif1= mouse 5T4 NP_035757, zwaif1a= NM_194392, z waif1b = XM_001338403, z waif1c = XP_001332297, z waif2 = XP_001337909, h waif2 = XP_497310, m waif2 = XP_486328. (D) Cartoon of the construct used to create the hsp70l:Axin1-YFP transgenic zebrafish line. Mouse Axin1 lacking the N-terminal RGS domain fused to EYFP at its C-terminus was cloned downstream of the zebrafish heat-shock protein 70-4 promoter. (E-G) Overexpression of Dkk1-GFP and Axin1-YFP recapitulates knockdown of wnt8 expression. (E) Classification of phenotypes at 24 hpf produced by axin1 RNA overexpression. Class I displays enlarged anterior neural structures including eyes (arrow) and reduced trunk and tail. Class II in addition shows signs of dorsalization (snailhouse-like tails). (F) Full-length zebrafish axin1, full-length mouse axin1 and mouse axin1 lacking the RGS domain produce similar phenotypes, but with different penetrance. (G) Comparison of phenotypes produced by knockdown of wnt8 (2 + 2 ng of wnt8 ORF 1 and wnt8 ORF 2 MO each) and by activating the heat shock inducible Dkk1-GFP and Axin1-YFP transgenes at early gastrula (50% epiboly) and organogenesis (22 hpf) stages. All embryos were heat-shocked for 1h at the stages indicated. Note that Axin-YFP overexpression phenocopies Dkk1-GFP, deleting posterior structures and increasing eye and forebrain size when activated during gastrulation, and inhibiting pectoral fin development when activated during organogenesis. Note also the very similar phenotypes produced by Dkk1-GFP or Axin1-YFP activation during gastrulation and that produced by Wnt8 MOs. (H-O) Zebrafish waif1a expression pattern determined by whole mount in situ hybridization at the indicated stages. ss = somite stage (K) Double staining with emx1, which marks the dorsal telencephalon, shows that the strongest waif1a expression in the CNS during somitogenesis is found in the forebrain-midbrain boundary and dorsal midbrain. (P-S) Expression pattern of Xenopus laevis waif1a.2 at the indicated stages. NF =Nieuwkoop and Faber. (T-U) Zebrafish waif1a expression is regulated by wnt8 during gastrulation. Dorsal views of embryos injected with 8 ng standard control morpholino or 4 ng each of wnt8 ORF 1 and wnt8 ORF 2 morpholinos are shown at 80% epiboly. 19/19 wnt8 morphant embryos showed severe downregulation of waif1a expression. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Waif1a acts as inhibitor of Wnt/β-catenin signaling in zebrafish embryos and HEK293T cells, related to figure 2. (A-B) Rescue of wnt8 induced anteroposterior patterning defects by waif1a is evident at early somitogenesis. (A) Classes of phenotypes produced by wnt8 RNA overexpression assayed at the 1 somite stage by whole mount in situ hybridization using the forebrain marker foxg1a (BF1, arrowhead), the midbrainhindbrain boundary marker her5 and the posterior mesoderm marker tbx6. Class 1, foxg1a reduction; class 2, reduction in length between anterior end of the embryo and the her5 domain; class 3, loss of foxg1a; class 4, radial expansion of tbx6. (B) waif1a RNA (60 pg) coinjection reduces the severity of phenotypes induced by 1.6 pg wnt8 RNA as assayed by the criteria defined in A and compared to embryos coinjected with equimolar amounts of Renilla Luciferase (Rluc) negative control RNA. *, p<0.05, Chi-Square test. (C) Overexpression of waif1a in zebrafish embryos interferes with wnt8-induced activation of a coinjected firefly luciferase reporter for β-catenin signaling. pBAR DNA (80 pg) plus 4 pg pGL4.73 hRLuc/SV40 was injected with 40 pg wnt8 or equimolar amounts of tomato control RNA. Firefly and renilla luciferase levels were determined during gastrulation. Reporter activity was normalized to renilla levels and is shown relative to tomato RNA control injected embryos. *, p<0.05, Student´s t-test. (D) Inhibitory ability of Waif1a towards different Wnt ligands in zebrafish embryos and HEK293T cells. n.d. = not done (E) Waif1a-GFP interferes with Wnt8-induced pBAR activation in HEK293T cells in a dosedependent manner. Firefly reporter activity normalized to levels of ubiquitously expressed Renilla luciferase are shown relative to vector transfected controls. +, 10 ng Waif1a-GFP, ++, 80 ng, +++, 160 ng. (F) Waif1a inhibits Wnt8-induced activation of another transcriptional reporter of β-catenin signaling, SuperTopflash. Firefly reporter activity normalized to levels of ubiquitously expressed Renilla luciferase are shown relative to vector transfected control cells. ***, p<0.001, Student′s t-test. (G-H) Waif1a reduces Wnt3a-induced stabilization of cytosolic β-catenin. (G) Total β-catenin was detected on Western blots of the cytosolic fraction of HEK293T cells 12h after stimulation with Wnt3a conditioned medium. (H) Quantification of data shown in (G). (I) Zebrafish waif1a MO1 is functional. Injection of waif1a morpholino 1 which is antisense to the waif1a 5′UTR suppresses translation of a reporter construct RNA containing the waif1a 5′UTR and GFP open reading frame. (J) waif1a knockdown sensitizes embryos to exogenous Wnt/β-catenin signaling. Embryos injected with the indicated reagents were scored for Wnt gain-of-function phenotypes as shown in Figure 2A. Note that embryos injected with 4 ng waif1a MO1 display significantly more severe phenotypes than control embryos in response to two doses (0.4 pg, ***, p<0.001, Chi-Square-test or 1.6 pg **, p<0.01) of wnt8 RNA. (K) Waif1/5T4 is expressed in HEK293T cells. Semiquantitative PCR for human waif1 and gapdh loading control on HEK293T cDNA and a positive plasmid control is shown. (L-M) Western blot (L) and quantitative real-time PCR (M) showing knockdown of endogenous human Waif1/TPBG/5T4 protein/RNA in HEK293T cells transfected with hwaif1 siRNA1 or hwaif1 siRNA2 96 h after siRNA transfection. (N) Direct Wnt/β-catenin target genes sp5l, cdx4 and vent are upregulated in embryos injected with 10 ng waif1a MO2. (sp5l 14/14, cdx4 13/13, vent 21/22 embryos). Note that the staining reaction was stopped before the wildtype expression pattern in control embryos became fully visible. |
|
Figure S5. The extracellular domain of Waif1a is required, but not sufficient for the inhibition of Wnt/β-catenin signaling, related to figure 5. (A) Zebrafish Waif1a deletion constructs tagged with GFP are expressed and correctly localized to the plasma membrane when injected as RNA into zebrafish embryos. EVL cells at sphere stage are shown. (B) waif1aΔC (200 pg) is equally potent as full-length waif1a (200 pg) in causing ventralization quantified with the dorsoanterior index when injected into the dorsal side of Xenopus embryos. *, p<0.05 Student′s t-test. (C) Zebrafish Waif1c-GFP does not inhibit Wnt8-induced pBAR activation in HEK293T cells, while Waif1a-GFP does. ** p<0.01, *** p<0.001 Student′s t-test. (D) Anti-GFP Western blot of lysates used for the experiment in C showing comparable expression levels of Waif1a-GFP and Waif1c-GFP. (E) A chimeric construct containing the Waif1a N-terminal extracellular and transmembrane domain and the Waif1c C-terminal intracellular domain (Waif1Na/Cc) tagged with Flag tag inhibits Wnt8- induced pBAR activity in HEK293Tcells, while a chimera of the Waif1c N-terminus with the Waif1a transmembrane domain and C-terminus (Waif1Nc/Ca) does not. ** p<0.01, *** p<0.001 Student′s ttest. (F) Anti-Flag Western blot of lysates used for the experiment in E showing similar expression levels of full-length Waif1a-Flag, Waif1Na/Cc-Flag and Waif1Nc/Ca-Flag. (G) Waif1Nc/Ca-Flag is correctly localized to the plasma membrane in HEK293T cells. (H) Media conditioned with a secreted version of the Waif1a extracellular domain (Waif1aN fused to the Fc fragment of human IgG, Waif1aN-IgG) cannot inhibit pBAR activation in HEK293T cells induced by Wnt3a CM, while media conditioned with Dkk1-IgG can. Western blots show that the CMs contained similar amounts of Waif1aN-IgG and Dkk1-IgG. **, p<0.01, ***, p<0.001 Student′s t-test. (I) The Waif1a transmembrane domain is not required for its function as inhibitor of Wnt/β-catenin signaling. A construct where the Waif1a transmembrane domain is exchanged with that of CD44 (Waif1a/CD44/Waif1a-GFP) is as potent as wild-type Waif1a-GFP in inhibiting Wnt8- induced pBAR activity in HEK293 cells. *** p<0.001 Student′s t-test. (J) waif1a inhibits activation of β-catenin signaling by Wnt-independent forced interaction of Frizzled and LRP receptors in zebrafish embryos. 85 pg of full-length waif1a-GFP RNA significantly reduced the phenotypes induced by 0.8 pg of dkk1fz5 RNA (***, p<0.001 Chi-Square test). In contrast, equimolar amounts of waif1aΔN-GFP RNA did not have any activity, while waif1aΔC-GFP efficiently rescued. Embryos were scored using the classification presented in Figure 2A. |
|
Waif1 does not interfere with LRP6-Frizzled8 interaction, but blocks Dkk1-dependent LRP6 internalization, related to figure 6. (A-B) The Waif1a extracellular domain (ED) does not directly bind the LRP6 ED and does not interfere with Wnt1-dependent interaction of the LRP6 ECD and Fzd8 CRD domain. (A) In mixtures of conditioned media, LRP6N-myc co-immunoprecipitates with Dkk1-IgG (lane 5), but not beyond background levels with Waif1aN-IgG (lane 6). (B) In mixtures of conditioned media, LRP6N-myc co-immunoprecipitates with the CRD domain of Fz8 in the presence (lane 3), but not the absence of Wnt1 (lane 2). Dkk1-Flag interferes with the LRP6- Fz8CRD interaction (lane 4), but Waif1N-GFP does not (lanes 5-6). (C) Waif1a does not block Wnt induced LRP6 phosphorylation. Overexpression of Waif1a-Flag in MEFs does not reduce Wnt3a-induced LRP6 phosphorylation, which is already apparent 15 minutes after stimulation with Wnt3a CM. (D) Human Waif1 inhibits pBAR activity in HEK293T cells induced by cotransfected Wnt8, but not by constitutive active LRP6ΔN, while dominant-negative TCF3 (ΔNTCF3-myc) can suppress LRP6ΔN induced pBAR activation. ***, p<0.001 Student′s t-test. (E-G) Waif1a specifically interferes with Dkk1-induced LRP6 internalization. (E) Waif1a interferes with Dkk1-mediated internalization of LRP6 (arrow). HEK293T cells stably expressing LRP6-GFP were transfected with mRFP-GPI or Waif1a-Cherry, incubated with Dkk1 conditioned media at 4°C, left there (upper panel) or shifted to 37°C and imaged 30 minutes later. Scale bar 10μm. (F) Quantification of Dkk1-induced LRP6 internalization data presented in K. n (cells with LRP6 puncta) = 360, n (mRFP-GPI) = 81, n (Waif1a-Cherry) = 12. ***, p<0.001 Chi-Square test. (G) Waif1a does not inhibit Clathrin-dependent internalization of Transferrin. HEK293T cells transfected with the indicated constructs were incubated with fluorescently labeled Transferrin for 10 min, washed and imaged. Note that treatment with the dynamin-inhibitor Dynasore blocked Transferrin uptake, while Waif1a-GFP did not. Scale bar 10μm. (H-I) Waif1a enhances localization of LRP6 to the plasma membrane in Xenopus animal cap cells, which express dkk1. (H) Apotome images of Xenopus animal cap explants injected with 60 pg lrp6-HA, together with 200 pg waif1a-GFP or in addition with 100 pg dkk1-Flag. Note increased membrane staining of LRP6-HA in the presence of waif1a, which is partly reversed by dkk1. (I) Quantification of LRP6 localization shown in L. (J) Waif1a and LRP6 compete for Dkk1 binding. Immunoprecipitation of LRP6-HA from HEK293T cells co-IPs Dkk1-Flag (lane 4), but much less so in the presence of Waif1a-GFP (lane 5). Conversely, significantly more Waif1a-GFP binds to LRP6-HA in the absence of Dkk1-Flag (compare lanes 5 and 6). |
|
Waif1a is a positive regulator of Wnt/PCP signaling, related to figure 7. (A-C) waif1a enhances wnt11 signaling. (A-B) Zebrafish embryos injected with 200 pg wnt11 RNA plus 60 pg RLuc or equimolar amounts (75 pg) of waif1a RNA photographed at 24 hpf. (C) wnt11 induced phenotypes were classified into 4 categories (not shown). Class I have hindbrain convergence defects, class 2 in addition cyclopia, class 3 the above plus shortened trunk/tail and class 4 lack recognizable trunk / tail structures. The phenotypes were counted in embryos injected with moderate (100 pg, left) or high (200 pg, right) doses of wnt11 RNA with or without 75 pg waif1a or 80 pg RLuc. waif1a RNA significantly enhanced moderate (*, p<0.05, Chi-Square test) and high doses (***, p<0.001) of wnt11. (D) waif1a genetically interacts with wnt5b. Images of the tail region of live zebrafish embryos at 24 hpf are shown. Embryos injected with 6 ng of waif1a MO1 plus 0.8 ng standard control MO have normal notochords (37/40 embryos). Likewise, low doses (0.8 ng) of wnt5b MO plus 6 ng control MO do not cause notochord defects (71/73). However, combined knockdown (6 ng waif1a MO1 plus 0.8 ng wnt5b MO) results in wavy notochords in 35/66 embryos. |
|
Waif1a enhances noncanonical effects of Dkk1, related to figure 8. (A) xwaif1 MO is functional. Injection of xwaif1a.2 MO (1.6 pmol) downregulates expression of a myc-tagged xwaif1a.2 construct harboring the endogenous xwaif1a.2 5′ UTR, while it has no effect on a xwaif1a.2-myc construct lacking the xwaif1a.2 5′UTR. (B) xdkk1 MO is functional. anti-XDkk1 antibody was used to detect levels of endogenous Xenopus Dkk1 protein in lysates of embryos injected with 1.6 pmol xdkk1 MO or control MO. (C) waif1a rescues CE defects induced by dkk1 knockdown (3 ng dkk1 MO) in zebrafish embryos. CE defects are evident by compressed myoD-positive somites (arrowheads) and widened krox20 and pax2.1 positive neural expression domains (brackets) at the 15 somite stage. Coinjection of 60 pg waif1a RNA completely rescues this phenotype (22/27 embryos). (D) Waif1a rescues body length defects caused by hypomorphic knockdown of zebrafish dkk1. Injection of 3 ng of dkk1 MO results in reduced body length (measured from the otic vesicle to the tip of the tail) at 24 hpf, which can be completely rescued by coinjection of 60 pg waif1a RNA. |
Reprinted from Developmental Cell, 21(6), Kagermeier-Schenk, B., Wehner, D., Ozhan-Kizil, G., Yamamoto, H., Li, J., Kirchner, K., Hoffmann, C., Stern, P., Kikuchi, A., Schambony, A., and Weidinger, G., Waif1/5T4 Inhibits Wnt/β-Catenin Signaling and Activates Noncanonical Wnt Pathways by Modifying LRP6 Subcellular Localization, 1129-43, Copyright (2011) with permission from Elsevier. Full text @ Dev. Cell