- Title
-
Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons
- Authors
- Tanaka, H., Maeda, R., Shoji, W., Wada, H., Masai, I., Shiraki, T., Kobayashi, M., Nakayama, R., and Okamoto, H.
- Source
- Full text @ Development
|
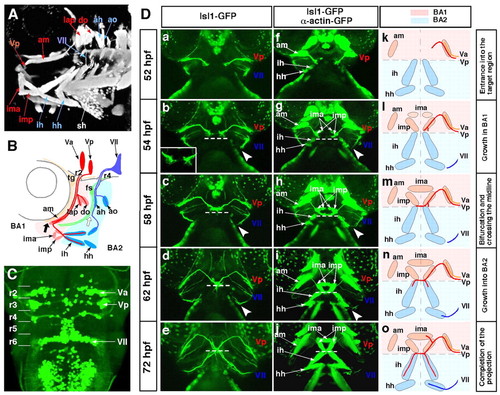
The stepwise axonal pathfinding of the Vp and VII motoneurons. The cranial axons and jaw muscles were visualised using the Isl1-GFP and α-actin-GFP double-transgenic strain. (A,B) The jaw region of a 72-hpf zebrafish embryo. Anterior, left. (B) Schematic of the projection pattern of the Va, Vp and VII motoneurons. Thick black and white arrows indicate the points at which these motor axons separate from the common pathways. (C) Dorsal view of the hindbrain of a 72-hpf Isl1-GFP transgenic zebrafish embryo. Anterior, top. The Va, Vp and VII motor nuclei are located in r2, r3 and r6, respectively. (Da-o) The stepwise outgrowth of the Vp (red) and VII (blue) motoneurons from 52-72 hpf. Ventral view; anterior, top. Broken lines indicate the boundaries between BA1 and BA2 (b-e,g-j). Arrowheads indicate the points at which the growth cones of the VII motoneurons stalled at 54-58 hpf (b-d,g-i). Va, anterior trigeminal motoneurons; Vp, posterior trigeminal motoneurons; VII, facial motoneurons; fs, facial sensory ganglion; tg, trigeminal sensory ganglion; BA1, BA2, first and second branchial arches; r, rhombomere; ah, adductor hyomandibulae; am, adductor mandibulae; ao, adductor operculi; do, dilatator operculi; hh, hyohyal; ih, interhyal; ima, intermandibularis anterior; imp, intermandibularis posterior; lap, levator arcus palatini; sh, sternohyoideus. |
|
No interaction is required between the left and right Vp motor axons for projection to the bilateral interhyal muscles in BA2. (A) Time-lapse images from 48-72 hpf of the Vp motoneurons after laser irradiation to the lateral part of the Vp motoneurons on the right side (a-e, arrowheads). Dorsal (a-e) and ventral (f-j) views of the same operated zebrafish embryo. Anterior, top. Brackets indicate remaining cells on the right side (a-e). Asterisks indicate the nerve end of the operated motoneurons (f-j). (B) Anterograde (a) and retrograde (b) labelling of the Vp motor axons of 72-hpf wild-type embryos with DiI. Ventral view; anterior, top. Asterisks indicate the points of DiI application. Arrowheads indicate the ends of the labelled axons. (C) Stochastic expression of GFP using the Isl1-GFP construct. Ventral (a) and dorsal (b) views; anterior, top. Arrowheads indicate a single Vp motoneuron labelled with the Isl1-GFP construct (a) and the ends of the labelled axons (b). |
|
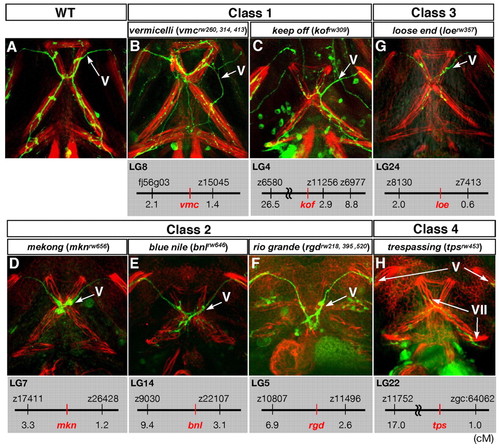
The projection patterns to the ventral jaw muscles of mutants displaying defects in the outgrowth of the Vp and VII motor axons. Ventral views of the lower jaw of 72-hpf wild-type (A) and the four classes of mutant (B-H) zebrafish embryos. Anterior, top. The cranial motor axons were labelled with the Isl1-GFP transgene, and each jaw muscle was stained with rhodamine-phalloidin. The genetic locus of each mutation is shown below each panel. |
|
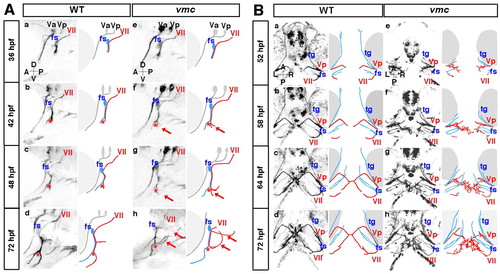
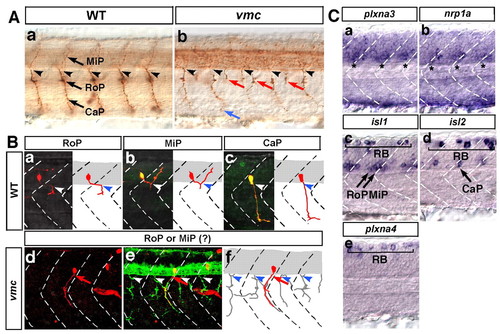
Time-lapse observations of the axonal outgrowth of the Vp and VII motoneurons in vmc embryos. (A) Time-lapse images of the axonal outgrowth of the VII motoneurons in wild-type (a-d) and vmc (e-h) zebrafish embryos. Lateral views; anterior, left. The axons of the VII motoneurons (red) and facial sensory (fs) neurons (blue) separated from each other at the points indicated by red asterisks (b-d,f-h). Arrows indicate the abnormal axons (f-h). (B) Time-lapse images of the axonal outgrowth of the Vp and VII motoneurons in wild-type (a-d) and vmc (e-h) embryos. Ventral views; anterior, top. The axons of the Vp and VII motoneurons (red) defasciculated into thin axons and grew abnormally (e-h). However, the axons of the trigeminal sensory (tg) and fs neurons (blue) grew normally (e-h). PHENOTYPE:
|
|
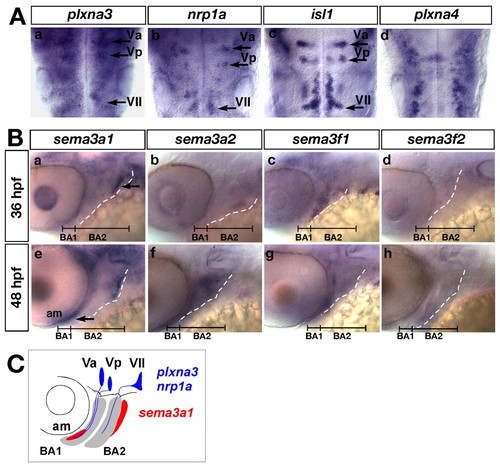
Expression of the zebrafish orthologues of plxna3, plxna4, nrp1a, sema3a and sema3f in the cranial region of zebrafish embryos. (Aa-d) Expression of plxna3, nrp1a, isl1 and plxna4 mRNA in the cranial motoneurons at 36 hpf. Dorsal views; anterior, top. (Ba-h) Expression of sem3a1, sema3a2, sema3f1 and sema3f2 mRNA in the jaw region. Lateral views; anterior, left. Broken lines indicate the posterior margin of BA2. Arrows indicate the expression domains of sema3a1. (C) Schematic of the expression patterns of plxna3, nrp1a (blue) and sema3a1 (red). |
|
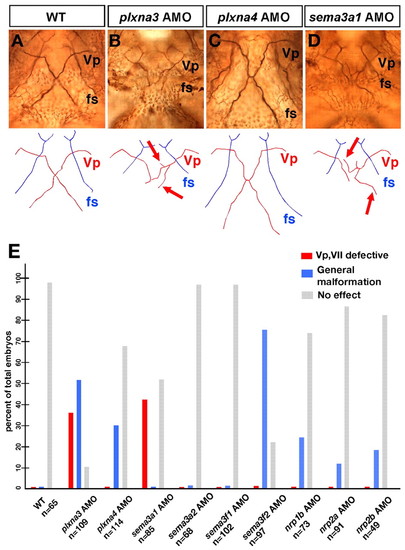
Plxna3 (vmc) is required for correct axonal pathfinding of the Vp and VII motoneurons. (A-D) Projection patterns of the Vp motoneurons in the wild-type embryo and the plxna3, plxna4 and sema3a1 morphants at 72 hpf. Ventral views; anterior, top. Arrows on schematics below indicate the abnormal trajectories of the defasciculated axons of the Vp motoneurons (B,D). (E) Quantitative data for phenotypes of the Vp and VII motoneurons observed in the morphants of the zebrafish orthologues of plxna3, plxna4, sema3a, sema3f, nrp1 and nrp2. PHENOTYPE:
|
|
Plxna3 (vmc) is required for correct axonal pathfinding of the primary motoneurons. (A) Axons of primary motoneurons of wild-type (a) and vmc (b) zebrafish embryos at 28 hpf. The blue arrow indicates an axon that showed abnormal pathfinding after extending from the normal exit point. Red arrows indicate axons that extended from the abnormal exit points. (B) Kaede-labelled primary motoneurons of wild-type and vmc Isl1-GFP embryos at 36 hpf (a-f, red). Arrows indicate the axons of RoP- or MiP-like neurons that extended out of the spinal cord through abnormal exit points (d-f). In e, all axons were visualised using a cocktail of znp-1 and zn-1 antibodies (green). Arrowheads in A and B indicate the normal exit points. (C) Expression of plxna3, nrp1a, isl1, isl2 and plxna4 mRNA in the spinal cord at 24 hpf. Asterisks indicate expression of plxna3 (a) and nrp1a (b) mRNA. Bracket (c-e) indicates Rohon-Beard sensory neurons (RB). In all images, dorsal is top, anterior is left. CaP, caudal primary motoneuron; MiP, middle primary motoneuron; RoP, rostral primary motoneuron. EXPRESSION / LABELING:
PHENOTYPE:
|
|
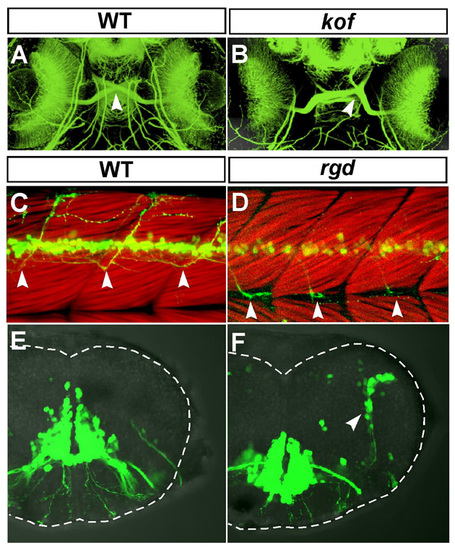
Additional defects in the kof and rgd mutant embryos in the developing central nervous system. (A,B) Ventral views of the head region of 72-hpf wild-type (A) and kof (B) embryos. Anterior, top. The retinal axons were stained with the anti-acetylated α-tubulin antibody. In the wild-type embryos, the optic chiasm was formed in the ventral midline (A, arrowhead). In the kof embryos, the position of the optic chiasm was randomly shifted toward the left or right near the midline (B, arrowhead). (C,D) Lateral views of the trunk region of 72-hpf wild-type (C) and the rgd (D) embryos. Dorsal, top; anterior, left. The trunk muscles were visualised with rhodamine-phalloidin. In the wild-type embryos, Isl1-GFP-positive spinal motor axons extended to the dorsal part of the trunk muscles (C, arrowheads). In the rgd embryos, Isl1-GFP-positive spinal motor axons grew ventrally and changed their growth pathway posteriorly after reaching the level of the myoseptum (D, arrowheads). (E,F) Transverse views of the hindbrain at r6 of the 72-hpf wild-type (E) and rgd (F) embryos. Dorsal, top. In the wild-type embryos, there were no Isl1-GFP-positive cells in the lateral part of the hindbrain (E). In the rgd embryos, Isl1-GFP-positive cells were ectopically observed in the lateral part of the hindbrain (F, arrowhead). PHENOTYPE:
|
|
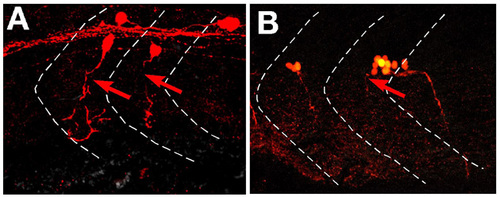
Abnormal axonal pathfinding of putative motoneurons in the spinal cord of vmc Isl1-GFP embryos at 36 hpf. (A,B) Dorsal, top; anterior, left. The axons of these neurons were visualised by stochastic expression of Kaede using the HuC-Kaede construct with direct fluorescent microscopy. Their axons grew anteriorly within the spinal cord, then extended out of the spinal cord through abnormal exit points (arrows). PHENOTYPE:
|
|
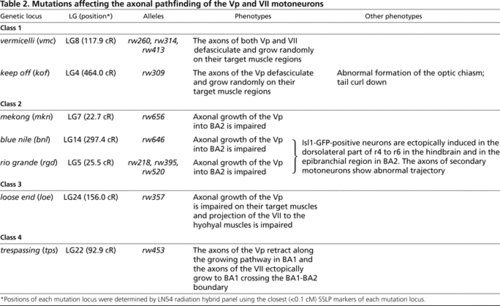
Mutations affecting the axonal pathfinding of the Vp and Vll motoneurons PHENOTYPE:
|