- Title
-
A zebrafish model of acmsd deficiency does not support a prominent role for ACMSD in Parkinson's disease
- Authors
- Fargher, E., Keatinge, M., Pearce, O., Piepponen, P., Panula, P., van Eeden, F.J.M., MacDonald, R.B., Bandmann, O.
- Source
- Full text @ NPJ Parkinsons Dis
|
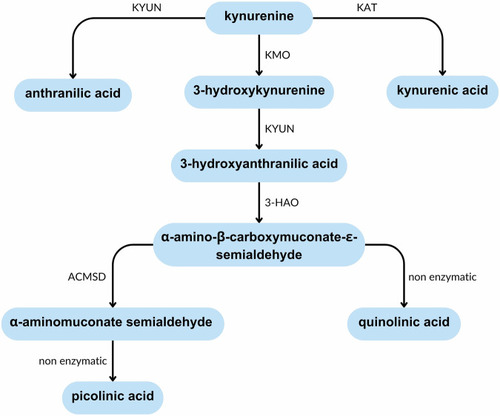
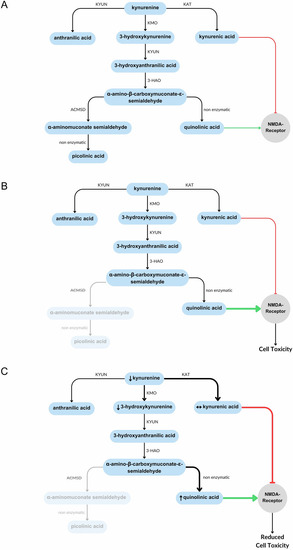
Summary of the kynurenine pathway. The kynurenine pathway is involved in the catabolism of tryptophan. The production of picolinic acid is considered an anti-inflammatory branch of the pathway, whist the production of quinolinic acid is considered pro-inflammatory due to its ability to activate immune cells and induce cell death. ACMSD acts at a branching point in this pathway that determines which of these products is created. 3-HAO 3-hydroxyanthranilate oxidase, ACMSD α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase, KAT kynurenine aminotransferase, KMO kynurenine-3-monooxygenase, KYUN kynureninase. |
|
|
|
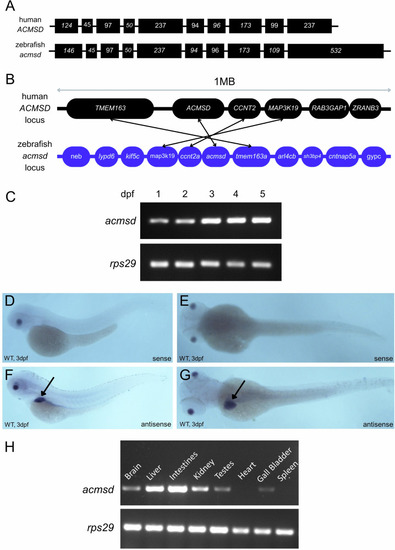
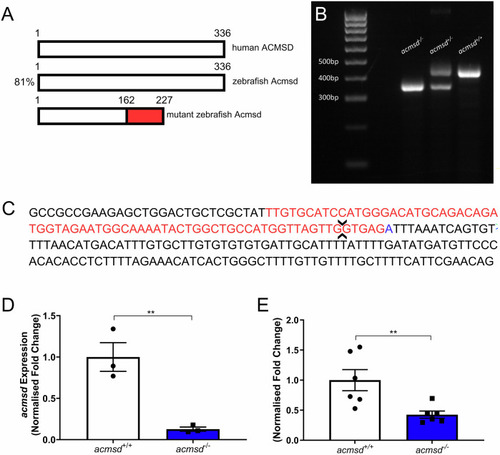
CRIPSR-generated mutation in |
|
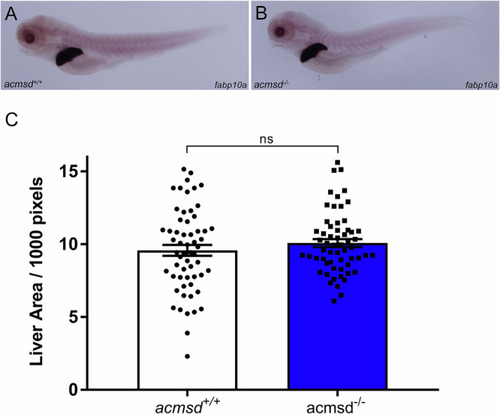
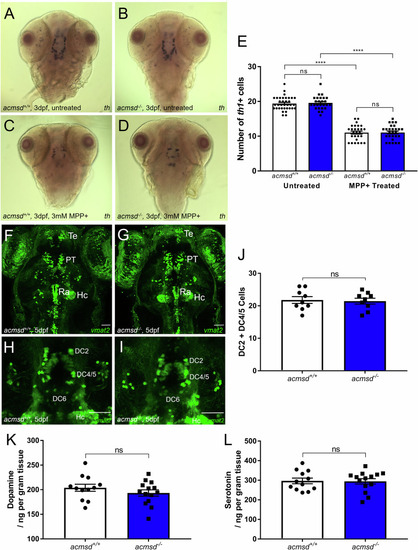
Liver size does not vary between 5dpf zebrafish underwent in situ hybridisation against fatty acid-binding protein 10a |
|
|
|
EXPRESSION / LABELING:
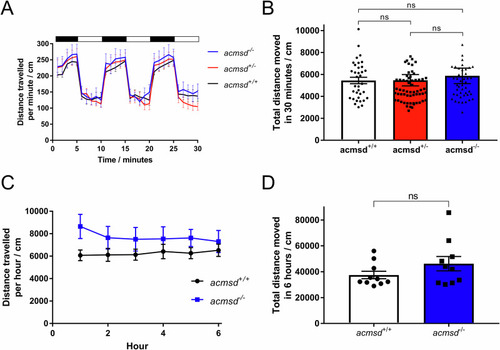
PHENOTYPE:
|
|
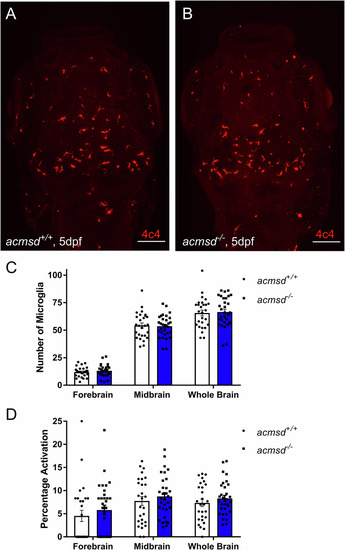
Representative images from wholemount immunohistochemistry against 4c4 in |
|
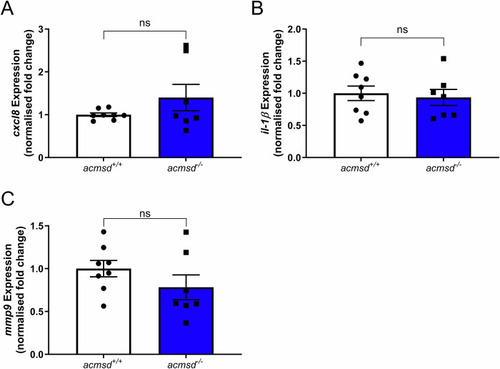
qPCR data suggested that genotype had no effect on the expression of EXPRESSION / LABELING:
|
|
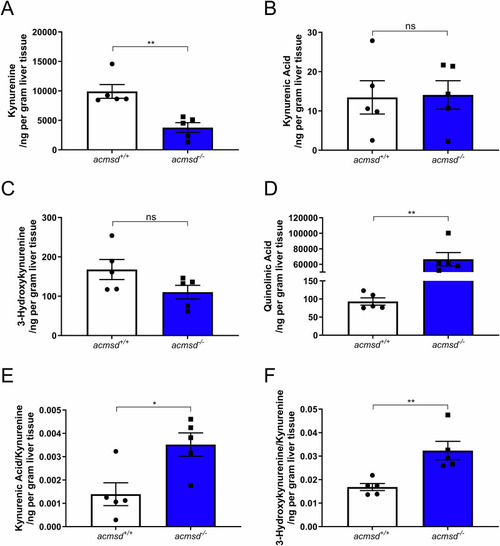
Kynurenine pathway metabolites were altered in In adult liver tissue, kynurenine was reduced in PHENOTYPE:
|
|
Summary of kynurenine pathway metabolite changes in |