- Title
-
Wnt/β-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish
- Authors
- Thorpe, C.J., Weidinger, G., and Moon, R.T.
- Source
- Full text @ Development
|
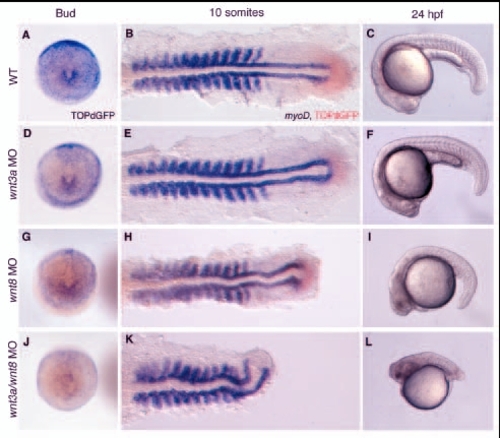
wnt3a and wnt8 coordinately regulate tail development. (A-C) Wild-type development. (D-L) Embryos from a transgenic TOPdGFP line were injected with wnt3a MOs (D,E,F), wnt8 MOs (G,H,I) or both (J,K,L). Expression of the ß-catenin-responsive reporter was examined at bud stage by in situ hybridization with a probe for GFP (A,D,G,J). Embryos are shown in a dorsal view of the tailbud, with anterior to the top. At the 10-somite stage (B,E,H,K), TOPdGFP expression was again assessed by in situ hybridization, in this case with a Fast Red color reaction. myoD expression, to visualize the defect in tail development, is in blue. Embryos are flat mounted and shown in a dorsal view with anterior to the left. At 24 hpf (C,F,I,L), living embryos of each type are shown in a lateral view, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
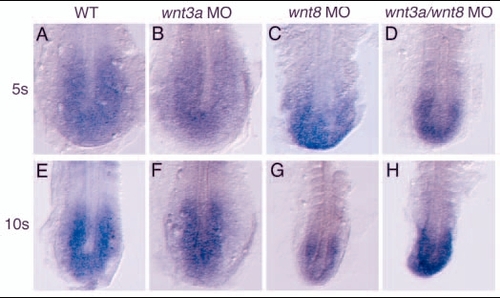
wnt3a and wnt8 are required for the maintenance, not initiation, of tailbud marker expression. (A-L) Embryos injected with the indicated morpholinos were fixed at tailbud stage and stained for ntl (A-D), fgf8 (E-H) and tbx6 (I-L). Embryos are shown in dorsal views, anterior to the top. (M-B') Injected embryos were allowed to develop until the 5-somite stage (M-P) or 10-somite stage (Q-B') and stained for tbx6 (M-T), spt (U-X) or ppt (Y-B'). All embryos are flat mounted and shown in dorsal views with anterior to the top. By the 10-somite stage in the tailbud of wnt3a/wnt8 MO embryos, tbx6 (T) and spt (X) are not expressed, while ppt (B') is still expressed at reduced levels compared with wnt3a MO (Z) or wnt8 MO (A'). EXPRESSION / LABELING:
|
|
Fgf signaling is not affected in the tailbud of wnt3a/wnt8 morphants. The expression of sprouty4 was examined at the 5-somite (A-D) and 10-somite (D-H) stages in wild-type (A,E), wnt3a MO (B,F), wnt8 MO (C,G), and wnt3a/wnt8 morphants (D,H). All embryos are shown in dorsal views of the tailbud, with anterior to the top. EXPRESSION / LABELING:
|
|
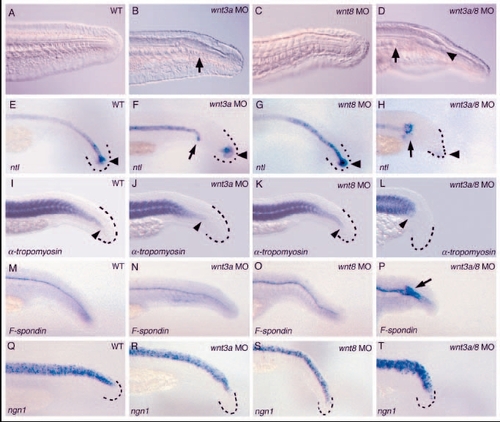
wnt3a and wnt3a/wnt8 morphants lack mesodermal fates at the expense of floor plate in the caudal tail. All embryos were examined at approximately 27-28 hpf. More mildly affected wnt3a/wnt8 morphants that made some tail structures were selected for these analyses. (A-D) Tails of living 28 hpf embryos, anterior to the left. Arrows in B and D indicate premature termination of the notochord in wnt3a and wnt3a/wnt8 morphants, respectively, and the arrowhead in D marks enlarged neural tube lumen. (E-P) Embryos were fixed at 26 hpf and stained with the indicated marker. The caudal tip of the tail is outlined in black dashes for clarity where necessary. (E-H) Embryos stained with ntl to visualize the notochord, arrowheads indicate ntl expression in the tailbud; arrows in F and H indicate the tip of the truncated notochord. (I-L) α-tropomyosin staining to visualize the somites; arrowheads mark the posterior extent of somite formation. (M-P) Embryos stained for F-spondin to visualize the floor plate; arrow in P indicates the expanded floor plate in wnt3a/wnt8 embryos. (Q-T) Pan-neural ngn1 expression in the neural tube is unaffected by wnt3a and wnt8 inhibition. |
|
sp5l transcription is induced by ectopic Wnt and repressed by interference with Wnt signaling. (A) Ectopic expression of sp5l at the animal pole of embryos injected with 10 pg wnt8 RNA, 6 hours after injection at shield stage (right panel, dorsal view), compared with wild-type expression in embryos injected with equimolar amounts of GFP RNA (7 pg, left panel). (B) Overexpression of dominant-negative TCF rapidly abolishes sp5l expression. Fish heterozygous for heat-shock-inducible dominant-negative TCF-GFP [Tg (hsp70:δTCF-GFP)w26] were outcrossed to wild type, the resulting embryos heat-shocked for 15 minutes or 30 minutes starting at the 1-somite stage and the whole clutch fixed immediately. In situ hybridization for GFP (light brown) in addition to sp5l (blue) was performed to identify transgenic embryos. sp5l expression is severely reduced in 100% (n=21) of transgenics 15 minutes after induction of the transgene (middle panel), and completely abolished in 100% (n=24) of transgenic embryos after 30 minutes (right panel). (C-F) Expression of sp5l in WT, wnt3a, wnt8 and wnt3a/wnt8 morphants at the 3-somite stage. EXPRESSION / LABELING:
|
|
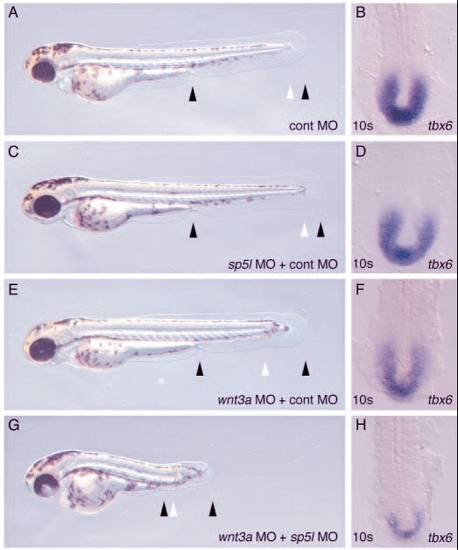
sp5l MO enhances wnt3a loss of function. Embryos were injected with 3.5 mg/ml control morpholino (A,B), 2.5 mg/ml sp5l MO + 1 mg/ml control MO (C,D), 1 mg/ml wnt3a MO +2.5 mg/ml control MO (E,F), or 1 mg/ml wnt3a MO + 2.5 mg/ml sp5l MO (G, H). (A,C,E,G) Live embryos are shown at 48 hpf, anterior to the left. (B,D,F,H) Embryos stained for tbx6 expression at the 10-somite stage and shown in a dorsal view of the tailbud region, anterior to the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
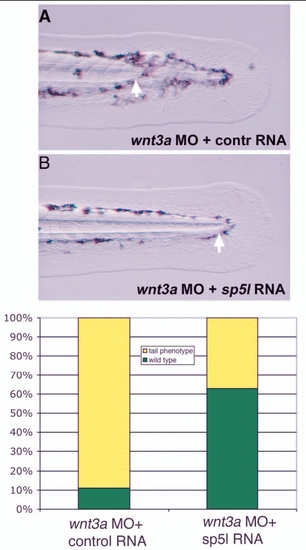
sp5l RNA overexpression rescues wnt3a morphants. Embryos are shown at 48 hpf, with arrows marking the posterior end of the notochord. (A) wnt3a MO embryos co-injected with 200 pg of a control RNA (renilla luciferase) typically have a truncated notochord (89%, n=104). (B) Most embryos co-injected with wnt3a MO and 200 pg sp5l MO have normal tail development (33% have truncated notochord; n=107). (C) Graph showing penetrance of rescue. |
|
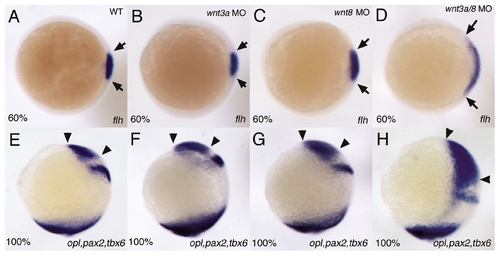
wnt3a MOs can enhance dorsal-ventral (D/V)and anterior-posterior (A/P) patterning defects associated with mild loss of wnt8 function. Embryos were injected with 2 mg/ml wnt3a MO (B,F), 0.25 mg/ml each of wnt8 ORF1 and ORF2 MOs (C,G), or both (D,H). (A-D) Animal pole views, dorsal to the right. Embryos were fixed at 60% epiboly and stained with flh to visualize the dorsal organizer, the limits of which are demarcated by arrows. wnt3a MO injection has no effect on the size of the organizer (B). At these doses, wnt8 MOs result in only a slight expansion of the flh domain (C), while injection of both morpholinos results in a significant expansion of the dorsal organizer (D). (E-H) lateral views, dorsal to the right. Embryos were fixed at 100% epiboly and stained with opl, to mark the prospective telencephalon, pax2, to mark the future midbrain/hindbrain boundary, and tbx6, to mark the margin. Neither wnt3a MOs (F) nor wnt8 MOs (G) caused any defects in A/P patterning of the neurectoderm, but wnt3a/wnt8 morphants showed a dramatic expansion of opl (H), reflecting the increased anterior character of the neurectoderm. With the higher doses of wnt8 MOs used for the other experiments in this paper, co-injection of wnt3a MOs does not enhance D/V and A/P patterning defects. EXPRESSION / LABELING:
|
|
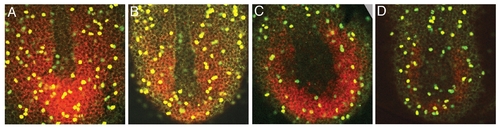
The rate of cell proliferation in the tailbud is unchanged in wnt3a/wnt8 morphants. Embryos were injected with 2 mg/ml wnt3a MO (B), 0.5 mg/ml each of wnt8 ORF1 and ORF2 MOs (C), or wnt3a, wnt8 ORF1 and wnt8 ORF2 MOs together (D), and fixed at the 4-somite stage, with uninjected embryos as the control (A). tbx6 expression was visualized with a Fast Red substrate for the color reaction, which yields a fluorescent (red) product. Anti-phosphohistone H3 antibody marks mitotic nuclei (green). To determine the mitotic index, we calculated the number of mitotic cells within the tbx6 expression domain, divided by the total number of cells within that domain. Three embryos were scored for each group of embryos. We calculated a mitotic index of 7.5 (n=1281) for wild type, 6.7 (n=1089) for wnt3a morphants, 7.5 (n=772) for wnt8 MO embryos and 6.8 (n=772) for wnt3a/wnt8 morphants. EXPRESSION / LABELING:
|
|
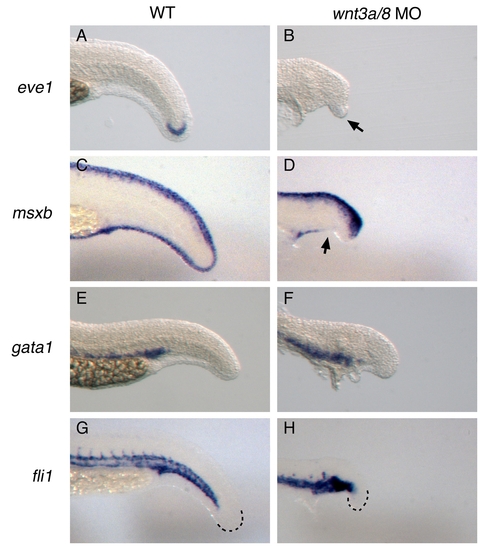
Examination of additional posterior fates in wnt3a/wnt8 morphants. Embryos were injected with wnt3a and wnt8 morpholinos. More mildly affected embryos that made some tail structures were collected and fixed at 27 hpf, along with uninjected wild-type embryos, and stained with the indicated marker. eve1 (A,B) was used as a general marker of the tailbud, msxb (C,D) was used to stain ventral tail fin, gata1 (E,F) was used to visualize the blood, and fli1 (G,H) was used to stain the developing vasculature. EXPRESSION / LABELING:
|