Fig. 6
- ID
- ZDB-FIG-250204-36
- Publication
- Sun et al., 2024 - Target protein identification in live cells and organisms with a non-diffusive proximity tagging system
- Other Figures
-
- Fig. 1
- Fig. 1 - Supplemental 1
- Fig. 1 - Supplemental 2
- Fig. 1 - Supplemental 3
- Fig. 2
- Fig. 2 - Supplemental 1
- Fig. 2 - Supplemental 2
- Fig. 2 - Supplemental 3
- Fig. 2 - Supplemental 4
- Fig. 3
- Fig. 3 - Supplemental 1
- Fig. 3 - Supplemental 2
- Fig. 3 - Supplemental 3
- Fig. 4
- Fig. 4 - Supplemental 1
- Fig. 4 - Supplemental 2
- Fig. 4 - Supplemental 3
- Fig. 4 - Supplemental 4
- Fig. 4 - Supplemental 5
- Fig. 4 - Supplemental 6
- Fig. 4 - Supplemetal 7
- Fig. 5
- Fig. 6
- Fig. 6 - Supplemental 1
- Fig. 6 - Supplemental 2
- Fig. 6 - Supplemental 3
- Fig. 6 - Supplemental 4
- Fig. 6 - Supplemental 5
- Fig. 6 - Supplemental 6
- Fig. 7
- Fig. 7 - Supplemental 1
- All Figure Page
- Back to All Figure Page
|
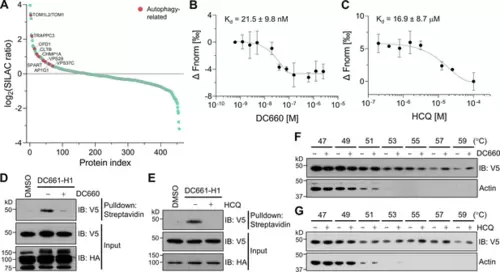
Target-ID for hydroxychloroquine (HCQ) by POST-ITDC661-H1. (A) Rank plot analysis of SILAC ratio values of proteins in three biological replicates. Autophage-related proteins ranked highly are marked in red. Heavy-labeled cells were treated with 200 nM DC661-H1, whereas light-labeled cells underwent incubation with both DC661-H1 (200 nM) and competitive DC660 (2 µM). (B, C) Microscale thermophoresis (MST) analyses demonstrate direct interactions of DC660 (B) and HCQ (C) with VPS37C, yielding Kd values of 21.5 ± 9.8 nM and 16.9 ± 8.7 µM, respectively. n = 2. Data are shown as mean ± s.d. (D, E) Immunoblot results indicate that the VPS37C-V5 protein from cells treated with DC661-H1 was significantly enriched after streptavidin pulldown. The competition between DC661-H1 and either DC660 (D) or HCQ (E) nearly completely abolished VPS37C binding. HEK293T cells were co-transfected with HA-Halo8KR-PafAS126A,K172R, SBPK4R-sPupK61R, and VPS37C-V5. After 24 hr, cells were incubated with 200 nM of DC661-H1, with or without 2 µM of DC660 (D) or HCQ (E). Twenty-four hours later, cells were collected for further analysis. (F, G) Cellular thermal shift assay (CETSA) results demonstrate that VPS37C becomes thermostable when exposed to DC660 (F) or HCQ (G). Selective stabilization of VPS37C is evident at temperatures of 53°C or higher, a phenomenon not observed in β-actin. |