Fig. 2
- ID
- ZDB-FIG-250204-18
- Publication
- Sun et al., 2024 - Target protein identification in live cells and organisms with a non-diffusive proximity tagging system
- Other Figures
-
- Fig. 1
- Fig. 1 - Supplemental 1
- Fig. 1 - Supplemental 2
- Fig. 1 - Supplemental 3
- Fig. 2
- Fig. 2 - Supplemental 1
- Fig. 2 - Supplemental 2
- Fig. 2 - Supplemental 3
- Fig. 2 - Supplemental 4
- Fig. 3
- Fig. 3 - Supplemental 1
- Fig. 3 - Supplemental 2
- Fig. 3 - Supplemental 3
- Fig. 4
- Fig. 4 - Supplemental 1
- Fig. 4 - Supplemental 2
- Fig. 4 - Supplemental 3
- Fig. 4 - Supplemental 4
- Fig. 4 - Supplemental 5
- Fig. 4 - Supplemental 6
- Fig. 4 - Supplemetal 7
- Fig. 5
- Fig. 6
- Fig. 6 - Supplemental 1
- Fig. 6 - Supplemental 2
- Fig. 6 - Supplemental 3
- Fig. 6 - Supplemental 4
- Fig. 6 - Supplemental 5
- Fig. 6 - Supplemental 6
- Fig. 7
- Fig. 7 - Supplemental 1
- All Figure Page
- Back to All Figure Page
|
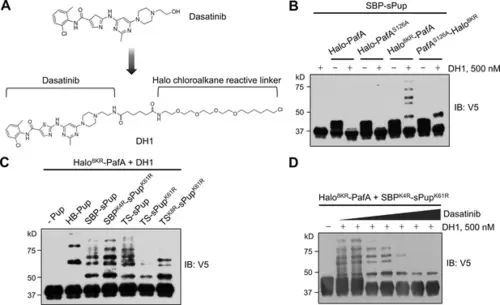
Optimized Halo-PafA and Pup substrates efficiently promote pupylation in vitro in a proximity-dependent manner. (A) The chemical structure of DH1, an HTL derivative of dasatinib. (B) Comparison of proximity-tagging by different Halo-PafA derivatives on in vitro pupylation. (C) Comparison of different Pup substrates on in vitro pupylation with 500 nM DH1. (D) Pupylation levels by DH1 decrease with an increasing amount of dasatinib as a competitor, ranging from 0.2 μM to 10 μM. (B–D) All reactions were conducted with 1 μM of a Halo-PafA derivative, 10 μM of one of the different Pup substrates, and 0.5 μM of a purified short SRC with a 2xV5 tag, SRC(247-536)-2xV5, at 37°C for 30 min. Pupylation levels of SRC were assessed by immunoblot (IB) using an anti-V5 antibody. |