Fig. 1 - Supplemental 1
- ID
- ZDB-FIG-250204-15
- Publication
- Sun et al., 2024 - Target protein identification in live cells and organisms with a non-diffusive proximity tagging system
- Other Figures
-
- Fig. 1
- Fig. 1 - Supplemental 1
- Fig. 1 - Supplemental 2
- Fig. 1 - Supplemental 3
- Fig. 2
- Fig. 2 - Supplemental 1
- Fig. 2 - Supplemental 2
- Fig. 2 - Supplemental 3
- Fig. 2 - Supplemental 4
- Fig. 3
- Fig. 3 - Supplemental 1
- Fig. 3 - Supplemental 2
- Fig. 3 - Supplemental 3
- Fig. 4
- Fig. 4 - Supplemental 1
- Fig. 4 - Supplemental 2
- Fig. 4 - Supplemental 3
- Fig. 4 - Supplemental 4
- Fig. 4 - Supplemental 5
- Fig. 4 - Supplemental 6
- Fig. 4 - Supplemetal 7
- Fig. 5
- Fig. 6
- Fig. 6 - Supplemental 1
- Fig. 6 - Supplemental 2
- Fig. 6 - Supplemental 3
- Fig. 6 - Supplemental 4
- Fig. 6 - Supplemental 5
- Fig. 6 - Supplemental 6
- Fig. 7
- Fig. 7 - Supplemental 1
- All Figure Page
- Back to All Figure Page
|
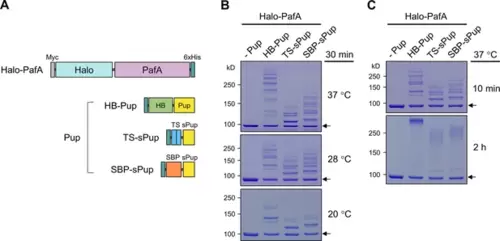
PafA fused with HaloTag exhibits high activity across a wide temperature range. (A) Schematic representation of Halo-PafA and three Pup substrates (HB-Pup, TS-sPup, SBP-sPup). HB, TS, and SBP refer to 6×His and BCCP, twin-STII (Strep-tag II), and streptavidin binding peptide, respectively. (B) In vitro self-pupylation of Halo-PafA (1 μM) with HB-Pup, TS-sPup, or SBP-sPup (10 μM) at 37°C, 28°C, and 20°C for 30 min. (C) Polypupylated Halo-PafAs were observed as high molecular weight bands after 2 hr pupylation reaction with three Pup substrates (HB-Pup, TS-sPup, SBP-sPup) at 37°C. Arrows indicate the Halo-PafA band without pupylation. |