- Title
-
Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish
- Authors
- Nechiporuk, A., Linbo, T., and Raible, D.W.
- Source
- Full text @ Development
|
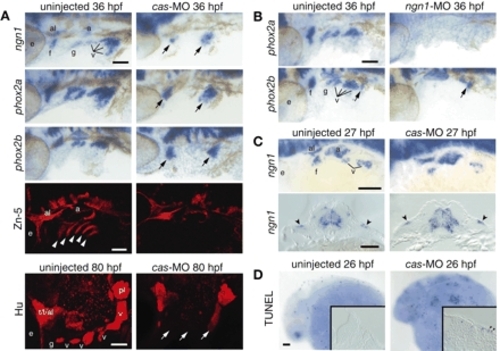
Endoderm is required for the neurogenesis and survival of EB placodes. Zebrafish embryos injected with a morpholino against cas (A,C,D, right) or ngn1 (B, right) and uninjected controls (A-D, left) were collected at various time points and processed for in-situ hybridization with ngn1, phox2a and phox2b riboprobes, immunolabeling with Zn-5 or Hu antibody, or TUNEL assay. All whole-mount panels show lateral views. Anterior is left. (A) Glossopharyngeal and small vagal placodes and ganglia are absent, while facial and large vagal placodes and ganglia are significantly reduced (arrows) after cas MO injection. Endodermal pouches are completely absent in cas morphants (Zn-5, white arrowheads). (B) phox2a and phox2b expression is absent in ngn1 morphants. phox2b-positive enteric neuronal precursors (arrow) were not affected by injection of ngn1-MO. (C) Transverse sections through the vagal placode revealed ectodermal expression of ngn1 (arrowheads), indicating that the remaining EB neurons in cas morphants derived from the placodes. (D) Endoderm is important for survival of the EB placode cells. Transverse section through the facial placode revealed TUNEL-positive cells in the cas morphants (arrowheads). Scale bars: 50 µm. a, acoustic ganglion; al, anterior lateral line ganglion; e, eye; f, facial placode or ganglion; g, glossopharyngeal placode or ganglion; pl, posterior lateral line placode or ganglion; t, trigeminal ganglion; v, vagal placode or ganglion. |
|
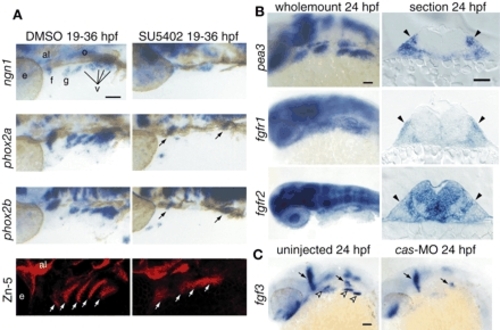
Fgf signaling is active in EB placodes. (A) Zebrafish embryos treated with the Fgfr inhibitor SU5402 (right) or DMSO (left) beginning at 19 hpf were collected at 36 hpf and processed for in-situ hybridization with ngn1, phox2a and phox2b. All panels show lateral views. Anterior is at right. In the SU5402-treated embryos, ngn1, phox2a and phox2b expression is either reduced (arrows) or absent. Fgfr inhibitor treatment also affected pharyngeal pouch morphology and size, as assessed by Zn-5 immunolabeling (white arrows). (B) Expression analyses of pea3, fgfr1 and fgfr2 at 24 hpf. Whole-mount panels are lateral views of the embryos; transverse sections are at the level of the facial placode. Note characteristic ectodermal thickenings, indicating cranial placodes (black arrowheads). pea3 and fgfr1 were expressed in the EB placodes, while fgfr2 was not. (C) fgf3 was expressed in the pharyngeal endoderm (left; empty arrowheads) at 24 hpf. As expected, the endodermal fgf3 expression was lost in cas morphants (right). Scale bars: 50 µm. Abbreviations are as in Fig. 1. |
|
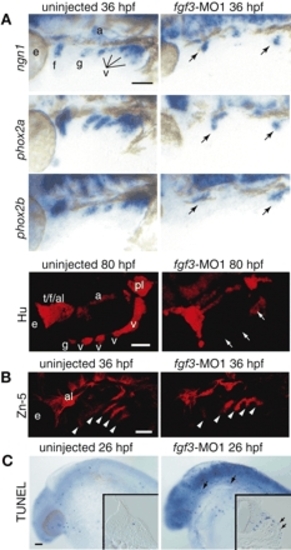
Fgf3 is necessary for inducing neurogenesis in EB placodes. Embryos injected with morpholino directed against fgf3 (A-C, right) and uninjected controls (A-C, left) were processed for in-situ hybridization with ngn1, phox2a and phox2b at 36 hpf, immunolabeling with Hu antibody at 80 hpf and Zn-5 antibody at 36 hpf, and TUNEL assay at 26 hpf. All whole-mount sections show lateral views. Anterior is left. (A) ngn1, phox2a, phox2b and Hu expression were absent in the glossopharyngeal and small vagal placodes and ganglia and significantly reduced in the facial and large vagal placodes and ganglia (arrows). (B) Zn-5 staining demonstrated that pharyngeal pouches were not affected by fgf3-MO1 injection (arrowheads). (C) TUNEL assay revealed dying cells in the ectoderm of the fgf3-morphants (arrows). Insets show transverse sections through the facial placode. Scale bars: 50 µm. Abbreviations are as in Fig. 1. EXPRESSION / LABELING:
|
|
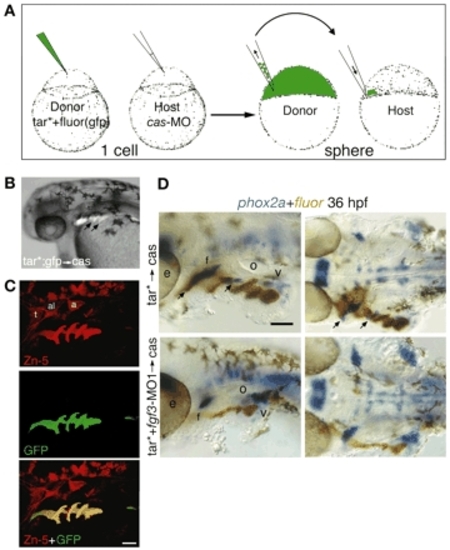
Pharyngeal pouch restoration is sufficient for rescuing phox2a-positive EB neurons. (A) Donor embryos were injected with a tar* mRNA and a lineage tracer (fluorescein or gfp), while host embryos were injected with cas morpholino. At sphere stage, 10-20 tar*-positive cells were transplanted to the margin of cas hosts and mosaic embryos were collected at 36 hpf. (B) Side view of the live cas-MO embryos that received tar* transplant. Arrows indicate endodermal pouches. (C) Lateral views of a cas host embryo that received tar*+gfp transplant. Zn-5 (red, top) and GFP (green, middle) were detected by immunolabeling. Note that tar*-positive cells efficiently rescue endodermal pouches (yellow overlay, bottom). (D) Side (left) and ventral (right) views of cas hosts that received tar*+fluorescein transplant with (bottom) or without (top) fgf3-MO1. Resulting embryos were processed to detect phox2a expression (blue) and fluorescein (brown) at 36 hpf. Note rescue of the glossopharyngeal and facial ganglia (arrows) in the embryos that did not receive fgf3-MO1 (top). By contrast, fgf3-MO1 tar*-transplanted cells failed to rescue EB ganglia (bottom). Scale bars: 50 µm. Abbreviations are as in Fig. 1. |
|
Neither endoderm nor Fgf3 is required for EB placode induction. Lateral views (A-D) and transverse sections (E-L) of embryos injected with cas (B,F,J) or fgf3 morpholino (C,G,K) or treated with SU5402 (D,H,L) or DMSO (A,E,I) beginning at 22 hpf are shown. Embryos were collected at 26 hpf and processed for in-situ hybridization with foxi1 riboprobe (A-H) or modified Richardson's stain (I-L). Panels E-G show sections through the vagal placode, while panels H-L show sections through the facial placode. EB placodes are marked by black arrowheads, while pharyngeal endoderm is marked by white arrowheads. Insets in E-H show magnified views of the placodes. Note ectodermal foxi1 expression and presence of the ectodermal thickenings in cas and fgf3 morphants, indicating presence of the EB placodes. foxi1 was not expressed in the ectoderm of the third and fourth arches (black arrows) in cas morphants. We consistently observed increased levels of the foxi1 transcript in fgf3 morphants (C,G). In addition, we observed nuclear fragmentation in EB placodes in cas and fgf3 morphants (empty arrowheads in J,K), indicating dying cells. While endodermal foxi1 expression was not affected, ectodermal expression was lost in the SU5402-treated embryos (D,H). Scale bars: 50 µm. e, eye; o, otic vesicle. EXPRESSION / LABELING:
|
|
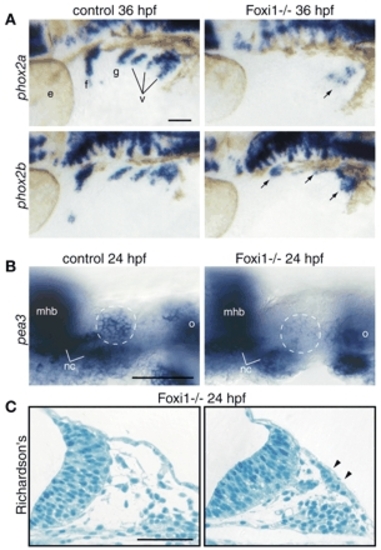
Foxi1 mediates Fgf response in the EB placodes. foxi1 mutant embryos and their wild-type sibs were processed for in-situ hybridization with phox2a and phox2b (A), pea3 (B) and modified Richardson's stain (C). All whole-mount panels show lateral views. Anterior is left. (A) When compared with their wild-type sibs (left), phox2a expression is absent in the facial, glossopharyngeal and small vagal ganglia, while it is significantly reduced in the large vagal ganglion (arrow) in the foxi1 mutants (right). By contrast, phox2b is still expressed in all EB ganglia, albeit at lower levels (arrows). (B) Optical sections through the facial placode (presumptive facial placode is outlined by a dashed circle). Note significant reduction of pea3 expression in the facial placode of the foxi1 mutant embryo (right), while other pea3 expression domains are unaffected. (C) Histological analysis of the transverse sections through the head region revealed the presence of columnar epithelium (marked by arrowheads; compare with the ectoderm immediately proximal to the facial placode, left panel), indicating presence of placodes in the foxi1 mutants. Scale bars: 50 µm. mhb, midhindbrain boundary; nc, chondrogenic neural crest of the first and second arches; o, otic vesicle. Other abbreviations are as in Fig. 1. |
|
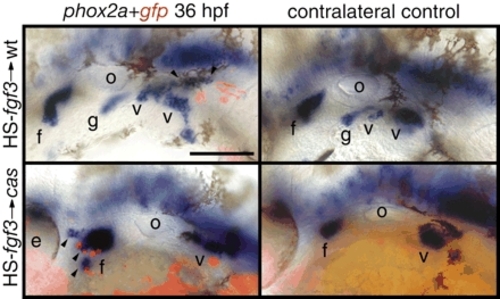
Fgf3 is sufficient to induce phox2a-positive epibranchial neurons. Zebrafish embryos were co-injected with HS-fgf3 and HS-gfp plasmids alone (top) or cas-MO (bottom) at the one-cell stage, heat shocked for 1 hour at 37°C beginning at 22 hpf and then assayed for phox2a expression (blue) and gfp expression (red) at 36 hpf. The majority of ectopic, phox2a-positive neurons (arrowheads) were immediately adjacent to the gfp-positive cells. Note significantly increased facial ganglion in cas morphant (bottom left) when compared with the contralateral control side. Anterior is at left. Scale bar: 50 µm. Abbreviations are as in Fig. 1. |

Unillustrated author statements EXPRESSION / LABELING:
|