- Title
-
Frataxin is essential for zebrafish embryogenesis and pronephros formation
- Authors
- Ercanbrack, W.S., Dungan, A., Gaul, E., Ramirez, M., J DelVecchio, A., Grass, C., Wingert, R.A.
- Source
- Full text @ Front Cell Dev Biol
|
An antisense morpholino oligomer targeting the exon 3 and exon 4 splice region is sufficient to completely knockdown |
|
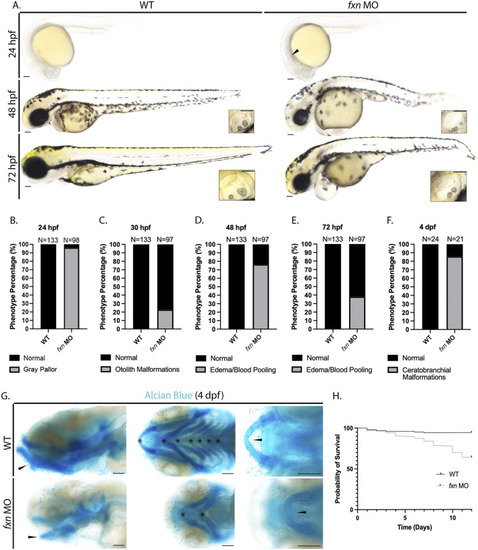
Morphological analysis of |
|
Pronephros segment development is significantly disrupted in EXPRESSION / LABELING:
PHENOTYPE:
|
|
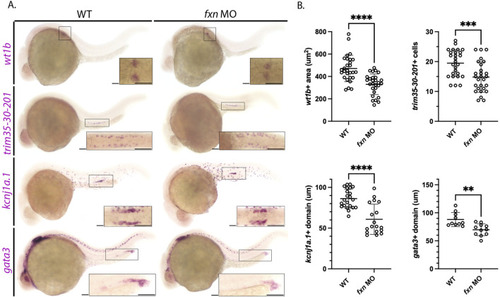
Further analysis of pronephros segment development in EXPRESSION / LABELING:
PHENOTYPE:
|
|
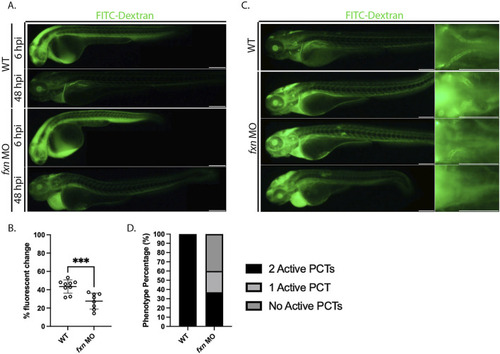
EXPRESSION / LABELING:
PHENOTYPE:
|
|
PHENOTYPE:
|
|
Cell death is elevated in |
|
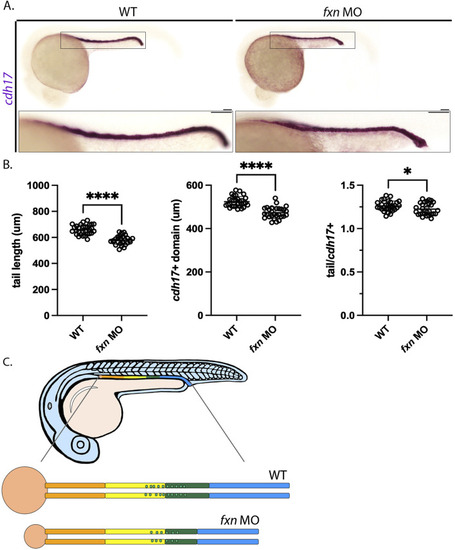
Tail/pronephros tubule ratio comparisons indicate that the embryonic kidney that develops within |