- Title
-
An Fgf-Shh signaling hierarchy regulates early specification of the zebrafish skull
- Authors
- McCarthy, N., Sidik, A., Bertrand, J.Y., Eberhart, J.K.
- Source
- Full text @ Dev. Biol.
|
The postchordal neurocranium requires Fgf signaling. (A-E) Wholemounted zebrafish neurocrania with the viscerocrania removed at 5 days post-fertilization. Anterior is to the left. (A) Wildtype, showing the prechordal versus postchordal regions of the neurocranium demarcated by a dashed line, (B) fgf8a mutant, (C) and fgf3 mutant embryos display normal neurocrania. (D) Variable neurocranial defects occur in 63% (n=5/8) of fgf8a-/-; fgf3+/- embryos (arrowheads). (E) The postchordal neurocranium in fgf8a-/-; fgf3-/- embryos is almost completely absent in 83% (n=5/6) of embryos, including the parachordals, anterior and posterior basicapsular commissure and the lateral commissures (arrowhead). abc-anterior basicapsular commissure, lc-lateral commissure, n-notochord, oc-occipital arch, pbc-posterior basicapsular commissure, pc-parachordals, prech.=prechordal neurocranium, postch.=postchordal neurocranium. Scale bar=20 µm. |
|
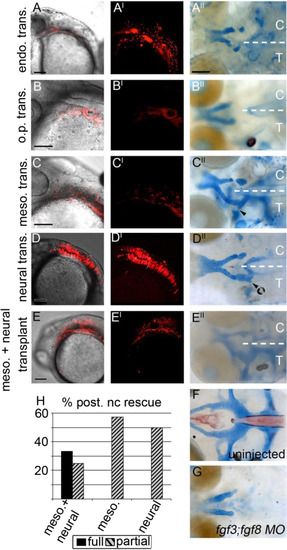
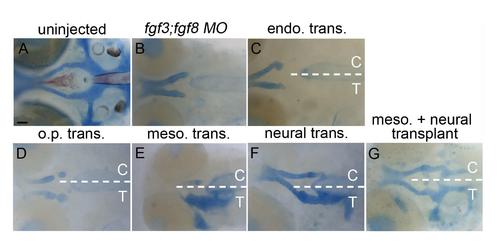
The mesoderm and neural ectoderm are Fgf sources required for postchordal neurocranial formation (A-E, A′-E′) 24 h post-fertilization confocal images of transplanted wildtype tissues into fgf3;fgf8 morpholino-injected hosts with and without DIC, respectively; (A′′-E′′) corresponding 4 days post-fertilization (dpf) neurocranial wholemounts with viscerocrania removed. Transplanted side of neurocrania is marked with a T, and control side with a C. (F and G) 4 dpf postchordal neurocrania of uninjected and fgf3;fgf8 morpholino injected,embryos respectively. Anterior is to the left. (A, A′ and A′′) Endoderm transplants, (B, B′ and B′′) otic placode transplants, (C, C′ and C′′) mesoderm only, (D, D′ and D′′) neural ectoderm only, and (E, E′ and E′′) mesoderm and neural ectoderm double-transplants. Endoderm and otic placode transplants fail to rescue the posterior neurocranium (compare A′′ and B′′ to F and G, n=0/12 for endoderm and n=0/9 for otic placode). Transplantation of mesoderm or neural ectoderm alone partially rescues the postchordal neurocranium (compare C′′ and D′′ to F and G, n=4/7 for mesoderm and n=8/16 for neural ectoderm). Transplantation of mesoderm and neural ectoderm together can fully rescue the postchordal neurocranium on the side of the embryo receiving the transplant (compare E′′ to F and G, n=4/12 for full rescue and n=4/12 for partial rescue). Quantification of partial or full rescue is shown in graph H. scale bars=100 µm in A-E and scale bar=20 µm in A′′. |
|
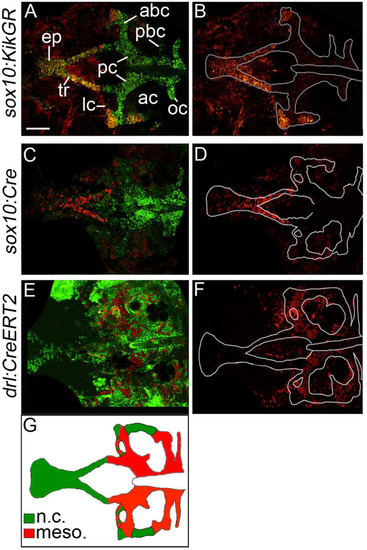
The zebrafish postchordal neurocranium is derived from both mesoderm and neural crest tissues. (A-F) Confocal images of 5 days post-fertilization flatmounted zebrafish neurocrania of (A and B) sox10:KikGR, (C and D) sox10:Cre;ubiRSG, and (E and F) drl:CreERT2;ubi:switch. Anterior is to the left. In C and D the red and green channels have been switched for clarity in comparisons to A and B. (B, D and F) Lineage-traced cells only, neurocrania outlined in white. (A-D) Neural crest contributes to the prechordal elements the ethmoid plate and trabeculae, as well as the lateral and anterior regions of the anterior basicapsular commissures and the lateral auditory capsules. (E and F) Mesoderm contributes the parachordals, anterior and posterior basicapsular commissure, the occipital arch, and the lateral commissures. Schematic of results shown in G (ne10 for each transgenic line). abc-anterior basicapsular commissure, ac-auditory capsule, ep-ethmoid plate, lc-lateral commissure, n-notochord, oc-occipital arch, pbc-posterior basicapsular commissure, pc-parachordals, tr-trabeculae. scale bar=50 µm. EXPRESSION / LABELING:
|
|
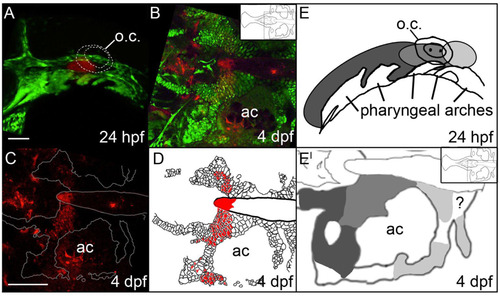
The anterior/posterior organization of postchordal cells is set by 24 h post-fertilization (hpf). (A-C) Confocal images of a Kaede-injected embryo at (A) 24 hpf and (B and C) 4 days post fertilization (dpf). Anterior is to the left. (A) At 24 hpf Kaede was photoconverted, shown in red (the remaining green Kaede is not evident due to the intense green fluorescence from the fli1:EGFP transgene), and (B) at 4 dpf, this same embryo shows labeling in the postchordal neurocranium (inset shows relative position in the neurocranium of magnified view). (C) Red channel only, the neurocrania is outlined in white. (D) Schematic of panel C, with red cells depicting the photoconverted region. (E and E′) Graphical representation of Kaede-mediated fate mapping at 24 hpf and 4 dpf. Inset in E′ shows relative position of magnified neurocrania. Question mark denotes a region that remained unlabeled in our analyses. ac- auditory capsule, o.c.-otic capsule. Scale bars=10 µm in A and 20 µm in C. |
|
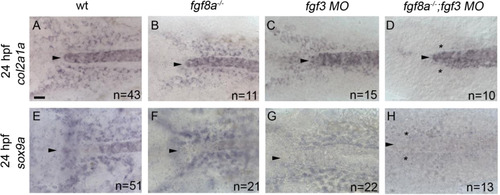
Cartilage and pre-cartilage markers col2a1a and sox9a are absent at 24 h post-fertilization (hpf) in fgf3;fgf8a knockdown embryos. (A-H) Images of 24 hpf embryos labeled with (A-D) col2a1a and (E-H) sox9a riboprobe. Dorsal view with anterior to the left, arrowhead denotes the anterior limit of the notochord. (A and E) Wildtype, (B and F) fgf8a mutant, and (C and G) fgf3 morpholino embryos display normal expression of both col2a1a and sox9a. However, (D and H) fgf3 morpholino-injected fgf8a mutants lose the expression of both col2a1a and sox9a (asterisk). scale bar=20 µm. |
|
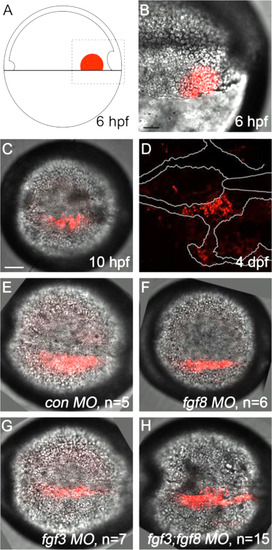
Kaede photoconverted endomesoderm postchordal-progenitor cells migrate appropriately in fgf3;fgf8a knockdown embryos. (A) Schematic of 6 h post-fertilization (hpf) zebrafish embryo showing the region of the embryo photoconverted in all subsequent experiments, dorsal to the right. (B-D) DIC confocal images showing the photoconverted area at 6 hpf (B, magnified region outlined in A), 10 hpf (C), and 4 days post-fertilization (dpf) (D). Cells have migrated adjacent to the notochord by 10 hpf and contributed to the postchordal neurocranium at 4 dpf. At 10 hpf, cells in (E) control, (F) fgf8, (G) fgf3, and (H) fgf3;fgf8 double morpholino-injected embryos are appropriately positioned (compared to C). scale bar=20 µm. |
|
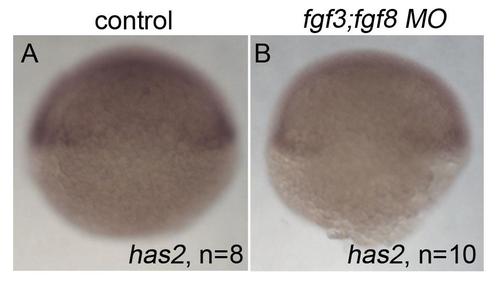
Loss of has2 in Fgf knockdown embryos contributes to the postchordal neurocranial loss phenotype. (A-D, A′-D′) Images of 10 h post-fertilization, and (E-F) 24 h post-fertilization embryos labeled with has2 riboprobe. Dorsal view and anterior to the left in all images. (G-J) Wholemounted zebrafish neurocrania with the viscerocrania removed at 5 days post fertilization. Anterior is to the left. (A′-D′) Magnified areas of region outlined in (A-D). (A and A′) Uninjected wildtype, (B and B′) fgf8a mutants, and (C and C′) fgf3 morpholino-injected wildtype display normal expression of has2, however, (D and D′) fgf3 morpholino-injected fgf8a mutants exhibit loss of expression of has2 in the presumptive postchordal neurocrania (D and D′). (E) At 24 hpf, some has2 expression remains along the notochord, however, (F) expression is lost in fgf8a;fgf3 double mutant embryos. Arrowheads denote end of the notochord. (G) Control morpholino-injected wildtype and (H) fgf8a mutants, as well as (I) has2 morpholino-injected wildtypes retain the postchordal neurocranium. However, (J) has2 morpholino-injected fgf8a mutants have a substantial loss of the postchordal neurocranium (arrowheads). n=notochord. scale bar=50 µm in A, 10 µm in A′ and E, and 20 µm in G. |
|
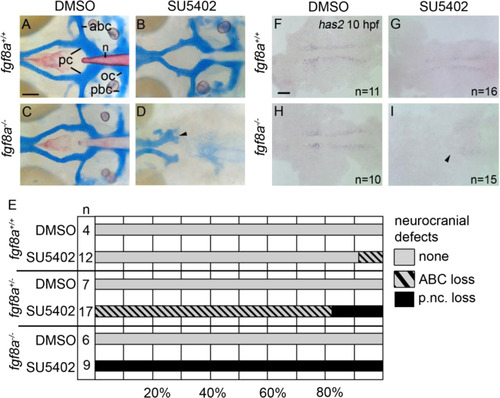
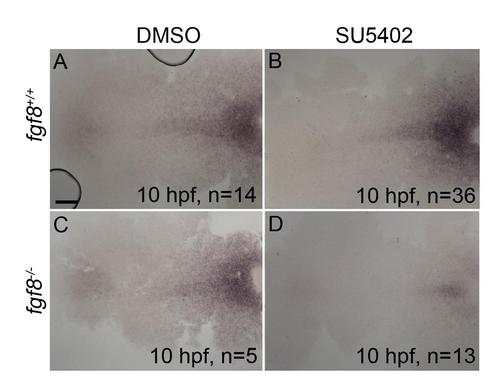
Postchordal neurocranial development requires Fgf signaling during gastrulation. (A-D) Wholemount zebrafish neurocrania, anterior is to the left. (A and B) Wildtype neurocrania treated with DMSO or SU5402 develop normally. (C) DMSO-treated fgf8a mutants are also unaffected, however, (D) those treated with SU5402 from 6 to 10 hpf develop severe postchordal neurocranial loss. (E) Quantification of postchordal neurocranial defects including none (see A), ABC loss, or complete postchordal neurocranial loss (p. nc. loss) in DMSO and SU5402 treated wildtype (fgf8a+/+), heterozygous (fgf8a+/-) and mutant (fgf8a-/-) embryos. (F-H) DMSO-treated wildtype and fgf8a mutants and SU5402-treated wildtype express has2 appropriately; however, (I) SU5402-treated fgf8a mutants display a loss of expression of has2 in postchordal neurocranial precursors (arrowhead denotes most anterior expression). abc- anterior basicapsular commissure, lc-lateral commissure, n-notochord, oc-occipital arch, pc-parachordals, pbc-posterior basicapsular commissure. scale bar=20 µm in A and scale 10 µm in F. |
|
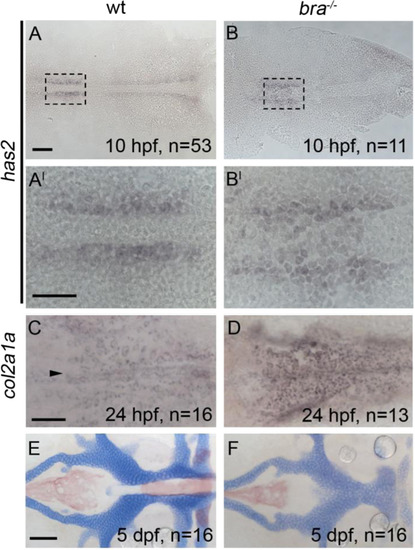
Postchordal neurocranial development does not require brachury function or a notochord. Anterior is to the left in all panels. (A-B, A′-B′) hyaluronan synthetase 2 (has2) is expressed in cephalic regions at 10 h post-fertilization (hpf) in both wildtypes and brachury mutants. (C and D) The late chondrogenic marker col2a1a at 24 hpf appears in both brachury mutants and siblings in the forming postchordal area of the developing embryo (compare D to C, arrowhead in C denotes anterior notochord). (E and F) At 5 days post-fertilization, postchordal neurocrania of brachury mutants are normal, sans notochord, compared to siblings (compare F to E). scale bar=50 µm in A and 10 µm in A′ and C and 20 µm in E. |
|
Chondrocyte differentiation is particularly sensitive to disruption of Hh signaling. All panels are anterior to the left. (A-B, A′-B′) At 10 h post-fertilization (hpf), the early mesoderm marker has2 is expressed in the anterior region of smoothened mutants and wildtypes (Compare B to A, B′ to A′). (C and D) However, smoothened mutants display a marked reduction in col2a1a expression in the forming postchordal neurocranium at 28 hpf compared to siblings (compare D to C, arrowhead denotes anterior notochord). Scale bar=50 µm in A and 10 µm in A′. |
|
Hh signaling is required after Fgf signaling for postchordal neurocranial development. The expression of has2 is retained following either (A-A′) DMSO or (B-B′) cyclopamine treatment from 6 to 10 h post-fertilization (hpf). (C and D and F-H) DMSO- and cyclopamine-treated embryos from 6 to 10 hpf show expression of both sox9a and col2a1a at 24 hpf, however, (E and H) cyclopamine-treated embryos from 10 to 24 hpf show a loss of sox9a and col2a1a expression in the postchordal neurocranium at 24 hpf. Arrowheads denote the anterior limit of the notochord. scale bar=50 µm in A and 10 µm in A′. |
|
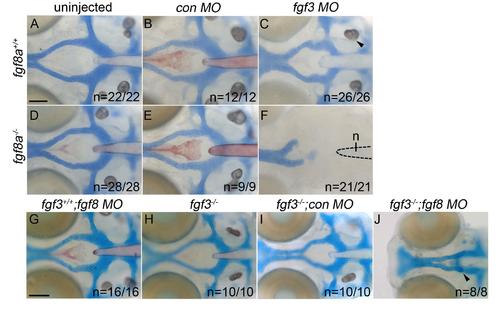
Loss of Fgf signaling causes postchordal neurocranial defects. (A-J) Wholemount zebrafish neurocrania at 5 days post fertilization, with the viscerocrania removed. Anterior is to the left. Wildtype embryos injected with (B) control or (C) fgf3-morpholinos develop normal posterior neurocranial structures compared to (A) uninjected controls. (D and E) Un-injected and control morpholino-injected fgf8a mutants display normal posterior neurocranial development compared to (A) wildtype controls, but (F) develop severe posterior neurocranial loss when injected with fgf3 morpholino. (G) Wildtype injected with fgf8 morpholino, (H) fgf3 mutants, and (I) control morpholino-injected fgf3 mutants display normal neurocranial development however, (J) fgf8 morpholino-injected fgf3 mutants display severe postchordal neurocranial scale bar=20 µm. |
|
Additional examples of transplants showing the mesoderm and neural ectoderm as Fgf sources required for postchordal neurocranial formation. (A-G) 4 days post fertilization (dpf) neurocranial wholemounts with viscerocrania removed. Anterior is to the left. Transplanted side of neurocrania is marked with a T, and control side with a C. (A and B) 4 dpf postchordal neurocrania of uninjected and fgf3;fgf8 morpholino injected embryos respectively. (C and D) Neither endoderm nor otic placode transplants, respectively, rescue postchordal neurocranial defects, however, (E and F) mesoderm or neural ectoderm transplants, respectively, can partially rescue postchordal neurocranial defects. (G) A dual mesoderm and neural ectoderm transplant can fully rescue the postchordal neurocranial defects found in fgf3;fgf8 morpholino-injected embryos. scale bar=20 µm. |
|
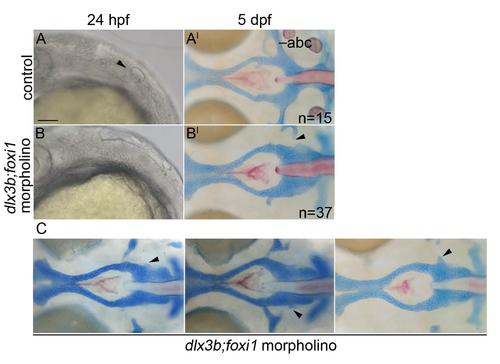
The postchordal neurocranium is not lost in dlx3b;foxi1 knockdown embryos. (A-B) DIC images of 24 hpf embryos and (A′-B′) the same embryo at 5 dpf wholemounted neurocrania with viscerocrania removed. Anterior is to the left. (A-A′) Un-injected control embryos show an otic placode at 24 hpf (arrowhead) and normal posterior neurocranium at 5 dpf. (B-B′) dlx3b;foxi1 double morpholino-injected embryos lack an otic placode at 24 hpf, but retain most of the posterior neurocranium at 5 dpf (arrowheads point to missing anterior basicapsular commissure). (C) Additional examples of dlx3b;foxi1 double morpholino-injected embryos at 5 dpf (arrowheads point to missing anterior basicapsular commissure). abc- anterior basicapsular commissure, scale bar= 20 µm. |
|
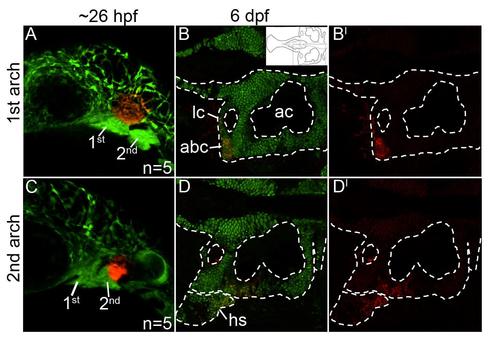
Mandibular- and hyoid-specific neural crest contributions to the postchordal neurocranium. (A-D′) Confocal images of sox10:kaede embryos at (A,C) 24 hours post fertilization and (B,B′, D, D′) 6 days post fertilization. Anterior is to the left in all images. (A) Photoconverted first arch neural crest cells, red fluorescence, contribute to the lateral anterior basicapsular commissure (B,B′, arrowhead, inset in B shows relative region shown in B and D). (C) Photoconverted second arch cells, red fluorescence, contribute to the lateral auditory capsule, with cells also labeled in the hyosymplectic, a second arch viscerocranial structure (D,D′). abc- anterior basicapsular commissure, ac- auditory capsule, hs- hyosymplectic, lc- lateral commissure. |
|
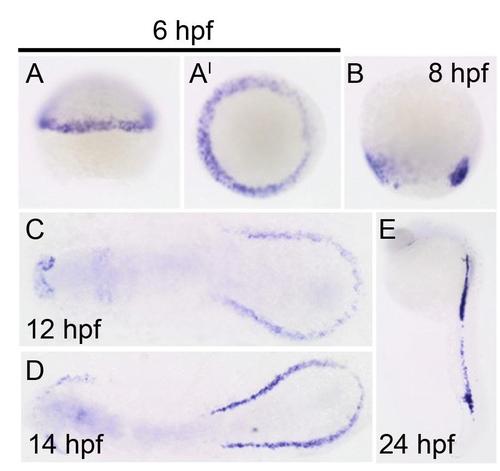
Cre expression in the drl:CreERT2 line confirms mesoderm-specific labeling at 6 hours post fertilization (hpf). All panels show Cre expression in drl:CreERT2 line at (A-A′) 6 hpf (lateral and dorsal views, respectively), (B) 8 hpf, (C) 12 hpf, (D) 14 hpf, and (E) 24 hpf. |
|
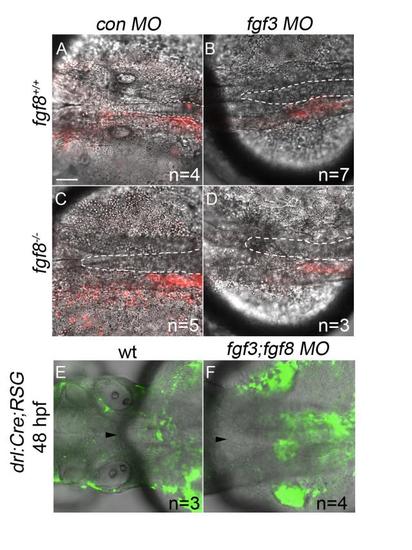
Postchordal-progenitor cells are present adjacent to the notochord at 24 and 48 hpf. (A-D) Confocal images of DIC and Kaede photoconverted postchordal-progenitor cells (in red) at 24 hpf. At 24 hpf, cells in (A) control morpholino- injected wildtype, (B) fgf3 morpholino-injected wildtype , (C) control morpholino-injected fgf8a mutant, and (D) fgf3 morpholino-injected fgf8a mutants are positioned appropriately next to the notochord. Dorsal view with anterior to the left. The notochord is outlined in a white dashed line. (E-F) Confocal and DIC images of tamoxifen-induced drl:Cre;ubi:RSG embryos of (E) wildtype and (F) fgf3;fgf8 MO-injected backgrounds at 48 hpf. Arrowhead denotes anterior notochord. Tamoxifen-induced conversion from red to green shows cells in (F) fgf3;fgf8 MO-injected embryos abutting the notochord at 48 hpf. scale bar= 20 µm. |
|
Expression of has2 at 6 hours post fertilization does not require fgf3;fgf8 function. (A,B) has2 expression at 6 hpf in (A) control and (B) fgf3;fgf8a morpholino-injected embryos. Dorsal view. |
|
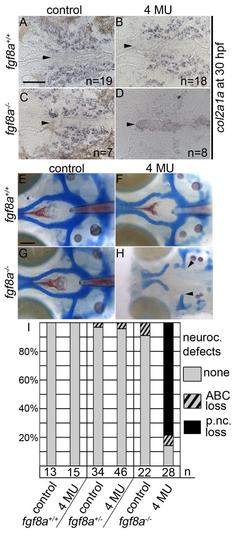
Inhibition of hyaluronic acid in fgf8a mutants causes postchordal neurocranial defects. (A-D) Images of 30 hpf embryos stained with col2a1a riboprobe. Dorsal view, with anterior to the left. (E-H) Wholemount zebrafish neurocrania at 5 days post fertilization, with the viscerocrania removed. Anterior is to the left. (A) Control-, (B) 4-MU-treated wildtype and (C) control-treated fgf8a siblings display normal col2a1a staining in the pre-postchordal neurocranium, however, (D) 4-MU-treated fgf8a mutants show a loss of col2a1a expression at the end of the treatment window (10-30 hpf). (E) Control- and (F) 4-MU-treated wildtype from 10 to 30 hours post fertilization, and (G) untreated fgf8a mutants have normal posterior neurocrania. However, (H) the mesoderm-derived regions of the neurocrania are lost in 4-MU treated fgf8a mutants (arrowheads). (I) Quantification of postchordal neurocranial defects including none (see E), ABC loss, or variable postchordal neurocranial loss (p. nc. loss) in control and 4-MU-treated wildtype (fgf8a+/+), heterozygous (fgf8a+/-) and mutant (fgf8a-/-) embryos. abc- anterior basicapsular commissure, n- notochord, oc- occipital arch, pc- parachordals, pbc- posterior basicapsular commissure. Scale bar= 50 µm in A, 20 µm in E. |
|
SU5402 treatment reduces expression of etv4. (A-D) 10 hpf etv4 in situ hybridization of (A) DMSO-treated wildtype, (B) 5 µM SU5402-treated wildtype, (C) DMSO-treated fgf8a mutant, and (D) 5 µM SU5402-treated fgf8a mutant, dorsal view, anterior to the left. Treatments from 6-10 hpf. Suboptimal dose of SU5402 reduces, but does not abolish, expression of etv4 in (B) wildtype embryos, with a further reduction in (D) SU5402-treated fgf8a mutants. scale bar= 10 µm. |
|
SU5402 treatment reduces the expression of col2a1a and sox9a in fgf8a mutants at 24 hpf. (A-D) col2a1a and (E-H) sox9a in situ hybridization at 24 hpf. Arrowheads denote anterior tip of notochord. Expression of col2a1a is observed abutting and along the notochord of (A) DMSO-treated wildtype (B) DMSO-treated fgf8a mutants, and (C) SU5402-treated wildtype. However, (D) SU5402-treated fgf8a mutants show a marked reduction in col2a1a expression. Expression of sox9a is observed abutting the notochord of (E) DMSO-treated wildtype (F) DMSO-treated fgf8a mutants, and (G) SU5402-treated wildtype. However, (H) SU5402-treated fgf8a mutants show reduced expression of sox9a. Dorsal view with anterior to the left. scale bar=20 µm. |
Reprinted from Developmental Biology, 415(2), McCarthy, N., Sidik, A., Bertrand, J.Y., Eberhart, J.K., An Fgf-Shh signaling hierarchy regulates early specification of the zebrafish skull, 261-77, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.