- Title
-
Zebrafish ambra1a and ambra1b Knockdown Impairs Skeletal Muscle Development
- Authors
- Skobo, T., Benato, F., Grumati, P., Meneghetti, G., Cianfanelli, V., Castagnaro, S., Chrisam, M., Di Bartolomeo, S., Bonaldo, P., Cecconi, F., Valle, L.D.
- Source
- Full text @ PLoS One
|
Ablation of ambra1 results in reduced birefringence in zebrafish embryos. (A). Representative images under normal and polarized light of 3-dpf live embryos injected with the indicated MOs. ATG-morphant embryos show reduced size, curved shape, pericardial edema and reduced birefringence when compared to WT and 5 m-control embryos. No visible abnormalities are evident in splice-morphants. The phenotypic defects of ATG-morphant embryos, including birefringence, are rescued by co-injection with 10 ng/embryo of human AMBRA1 mRNA. (B). Quantification of embryo trunk muscles birefringence shows a severe and statistically significant reduction in ambra1 ATG- and in co-injected morphants. The birefringence is faintly reduced in ambra1 splice-morphants, whereas WT and 5 m-control embryos display highly birefringent skeletal muscles. Muscle birefringence is statistically increased when ATG- and co-injected morphants are co-injected with human AMBRA1 mRNA (***, P<0.001). PHENOTYPE:
|
|
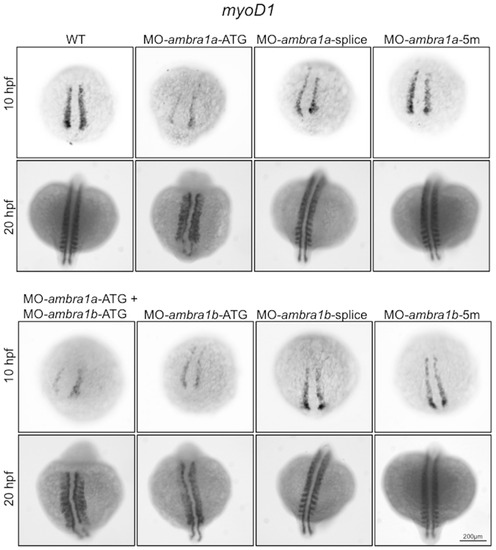
In situ hybridization analysis of myoD1 expression in ambra1 knockdown embryos. Expression of myoD1, analyzed in embryos injected with the indicated MOs at 10 and 20 hpf, is affected in ambra1 ATG-morphants and in co-injected morphants. No differences are evident in ambra1 splice-morphants when compared to WT and 5 m-control embryos. Embryos are shown by dorsal view, anterior side on the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Abnormal morphology of ambra1 knockdown embryos, as revealed by haematoxylin/eosin staining. Representative longitudinal sections of 3 dpf control and ambra1 morphant embryos. Myofibers of ATG- and co-injected morphants muscles are highly disorganized and display irregular myosepta boundaries. The phenotype of splice-morphants is much less severe when compared to WT and 5 m-control embryos. PHENOTYPE:
|
|
Abnormal morphology of ambra1 knockdown embryos, as revealed by toluidine blue staining. Representative longitudinal and cross sections of 3 dpf control and ambra1 morphant embryos. Muscles of ambra1 ATG-morphants show a severe phenotype, with misaligned myofibers scattered in the somitic compartment. Black arrows, areas devoid of staining; white arrows, large myonuclei with condensed chromatin; black arrowheads, interruption of myoseptum; asterisks, opaque material replacing lost myofibers. PHENOTYPE:
|
|
Cell proliferation in muscles of 3 dpf control and ambra1 morphant embryos. Mitotic cells, detected by immunostaining for phospho-histone H3 in longitudinal sections, are more abundant in ATG-morphant embryos with respect to WT and 5 m-control embryos. Anterior is to the left and dorsal up. PHENOTYPE:
|
|
Pax7 expression in control and ambra1 morphant embryos. Lateral views of 2 dpf muscles analyzed by immunofluorescence for Pax7. In WT and 5-control embryos, Pax7-positive cells are localized at the edge of somites, whereas in ambra1 morphants many Pax7-positive cells are misplaced in the spaces between myosepta. Anterior is to the left and dorsal up. |
|
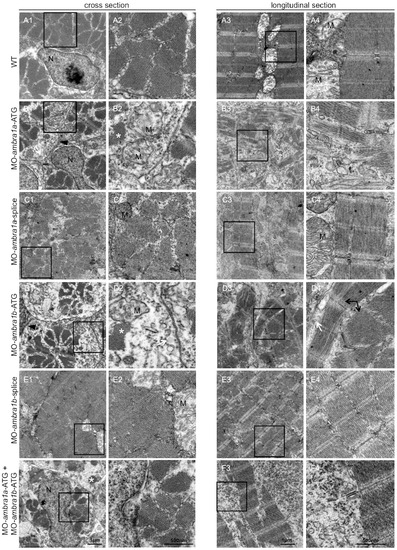
Ultrastructural analysis of ambra1 morphants muscles reveals disorganized sarcomeres. Representative electron micrographs of cross and longitudinal sections of 3 dpf (WT, panels A1–A4), ambra1a ATG-morphant (panels B1-B4), ambra1a splice-morphant (panels C1–C4), ambra1b ATG-morphant (panels D1–D4), ambra1b splice-morphant (panels E1-E4), and co-injected morphant (panels F1–F4) zebrafish embryos. Columns 2 and 4 show higher magnification views of the boxed areas in column 1 and 3, respectively. Muscles of WT and 5 m-control (not shown) embryos display well-organized myofibers, showing densely packed sarcomeres with regular organization of thin and thick myofilaments. ambra1 depleted muscles show a number of ultrastructural defects, with small patches of disorganized myofibers and mitochondria scattered throughout the cytoplasm. Black arrows, area with myofibrils having different orientations; white arrow, dilated sarcoplasmic reticulum not in contact with myofibrils; asterisks, fragments of torn myofibrils; M, mitochondria; N, nucleus. PHENOTYPE:
|
|
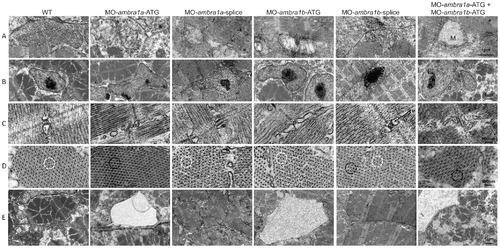
Ultrastructural analysis of ambra1 morphants muscles reveals disorganized subcellular structures. When compared to WT and 5-control (not shown) embryos, ambra1 morphant embryos display alterations of mitochondria (row A), nuclei (row B) and triads (row C), perturbation of the hexagonal arrangement of thick and thin filaments (row D), and dilations of the endoplasmic reticulum (row E). Muscles of morphant embryos show the presence of areas with reduced thin filaments (black circles in row D) adjacent to more normal-appearing hexagonal structures (white circles in row D). Co-injected double morphant embryos show exacerbated defects of these structures, whereas defects are barely evident in splice-morphants. PHENOTYPE:
|
|
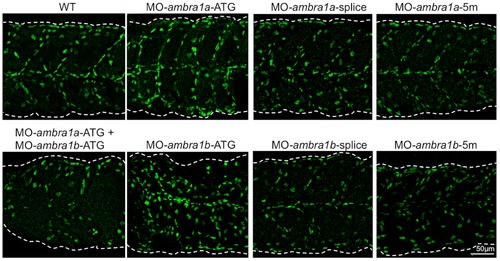
Knockdown of ambra1 interferes with myosin organization in slow and fast muscles and with myosepta. Lateral views of 3 dpf embryos labeled with the F59 antibody (slow muscle fibers) and with the F310 antibody (fast muscle fibers), showing abnormally organized myofilaments in ATG-morphant embryos when compared to WT and 5 m-control embryos. Slow fibers are thinner in ATG-morphant embryos, whereas fast fibers of splice-morphant embryos display a wavy phenotype, visibly different from controls. The asterisks indicate broken or missing muscle fibers. Laminin labeling highlights the loss of the V-shape arrangement of somites and reveals interrupted myosepta (arrowheads) in ATG-morphant embryos. Defects in myosin organization and myosepta structure are rescued with the by co-injection with human AMBRA1 mRNA. Anterior is to the left and dorsal up. PHENOTYPE:
|
|
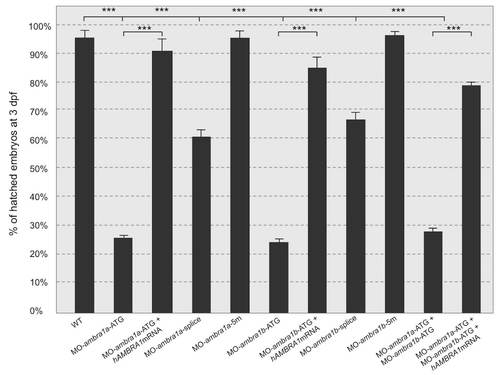
Quantification of chorion hatching in 72 hpf embryos after injection with the different MOs. PHENOTYPE:
|
|
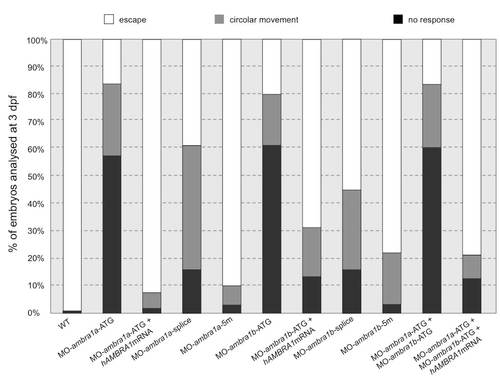
Quantification of touch-evoked response and circular movement at 3 dpf. Embryos were quantified in three independent microinjections, and the number of embryos for each microinjection experiments was about 80. PHENOTYPE:
|
|
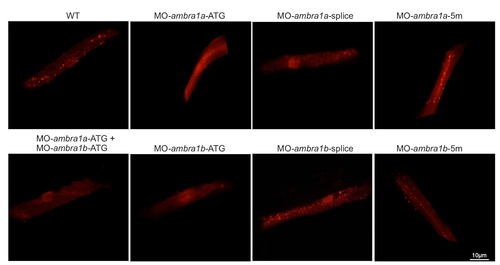
Fluorescence detection in muscle fibers of control and ambra1 morphant embryos following transfection with a Lc3-RFP reporter construct. Several fluorescent puncta have been detected in the transfected muscle fibers from WT and 5m-morphant embryos whereas only few puncta were visibile in ATG-morphant embryos. |