- Title
-
Prdm14 acts upstream of islet2 transcription to regulate axon growth of primary motoneurons in zebrafish
- Authors
- Liu, C., Ma, W., Su, W., and Zhang, J.
- Source
- Full text @ Development
|
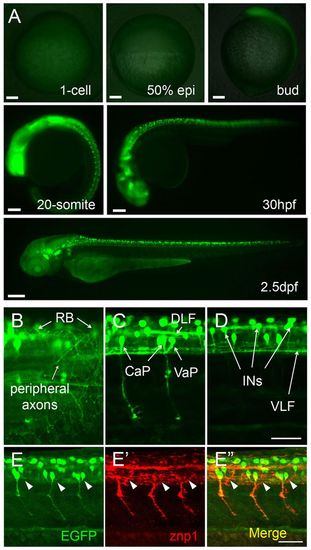
Neuron-specific expression of GFP transgene in slg+/- zebrafish embryos. (A) GFP expression starts from the bud stage and is restricted to a subset of embryonic neurons. (B-D) GFP expression in RB, CaP, VaP and INs at 32 hpf. (E-E′′) CaP axons labeled by znp1 antibody staining are GFP positive in 26-hpf embryos, whereas MiP axons (arrowheads) are GFP negative. All images are lateral views. Animal pole is to the top for 1-cell and 50% epiboly; dorsal to the right for bud and 20-somite; dorsal to the top and anterior to the left for 30 hpf and 2.5 dpf in A and for B-E′′. CaP, caudal primary motoneurons; DLF, dorsal longitudinal fasciculus; INs, interneuron-like cells; RB, Rohon-Beard neurons; VaP, variable primary motoneurons; VLF, ventral longitudinal fasciculus. Scale bars: 100 μm in A; 50 μm in B-E′′. |
|
prdm14 is trapped in the slg mutant. (A) Boundary sequences of the inserted transposon and prdm14 gene. (B,C) Zebrafish Prdm14 protein contains a SET domain and a six zinc-finger domain, which are highly conserved. z, zebrafish; m, mouse; h, human. (D) PCR genotyping confirms that the prdm14 gene is disrupted in slg mutants (right). Heterozygotes and homozygotes show different GFP intensities (left). (E-M) prdm14 RNA expression patterns detected by in situ hybridization. prdm14 is expressed in neural plate at 30% epiboly (E) and in the anterior neural plate at 75% epiboly and bud stages (F,G). At the 1-somite stage, prdm14 RNA is restricted to dorsal and ventral neuron precursors and precordal plate (H,K) and at the 10-somite stage prdm14 is detected in neuron precursors of the spinal cord (I,L). At the 22-somite stage prdm14 is further restricted to the ventral midbrain, olfactory sensory neurons, statoacoustic neurons, trigeminal neurons and spinal cord (J,M). (N-O′′) prdm14 RNA is co-expressed with ngn1 at the 3-somite stage as detected by double in situ hybridization. (P-P′′) prdm14 is co-expressed with islet2 in RB and CaP at the 26-somite stage. Animal view, dorsal to the top in E-G; anterior view, dorsal to the top in H-J,N-N′′; dorsal view, anterior to the left in K,L; lateral view, anterior to the left in M,P-P′′; dorsal view, anterior to the bottom in O-O′′. N, neural plate; AN, anterior neural plate; V and D, ventral and dorsal neuron precursors; PP, precordal plate; SC, spinal cord; M, ventral midbrain; O, olfactory sensory neurons; S, statoacoustic ganglion neurons; T, trigeminal neurons. Scale bars: 500 μm in D; 200 μm in E-P′; 100 μm in N′′-P′′. EXPRESSION / LABELING:
|
|
prdm14 expression is reduced in slg mutant and splmo morphant embryos. (A) The zebrafish prdm14 genomic locus. The transposon is inserted in the first intron. Primers used in C and D are indicated. (B,C) RNA in situ hybridization (B, arrowheads) and RT-PCR (C) indicate that prdm14 expression is greatly reduced in slg mutant embryos. ODC, ornithine decarboxylase 1 internal standard. (D) prdm14 splicing is disrupted in splmo morphants. (E) Real-time RT-PCR results indicate that prdm14 expression is reduced to ~75% in slg heterozygotes and to ~20% in homozygotes. Error bars indicate s.e.m. of triplicate experiments. (F) Immunoprecipitation (IP) showing that Prdm14 protein levels are greatly decreased in slg homozygotes and splmo morphants. Scale bar: 500 μm. |
|
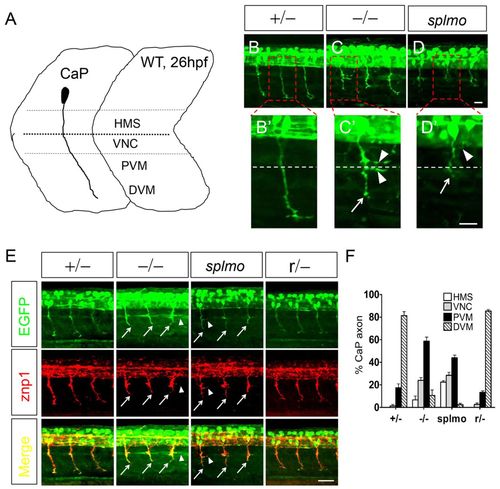
CaP axons are shortened in slg mutant and splmo morphant zebrafish embryos. (A) CaP axon projection in 26-hpf wild type. Four regions are defined to describe CaP axon outgrowth: HMS, horizontal myoseptum; VNC, myotome adjacent to the ventral edge of the notochord; PVM and DVM, proximal and distal portion of the ventral myotome. (B-D2) slg mutant and splmo morphant embryos show greatly shortened CaP axons at 26 hpf. The boxed regions are magnified in B2-D2. Dashed line indicates horizontal septum. (E) znp1 immunostaining shows that the shortened axons are CaP in slg mutant and splmo morphant embryos. (F) Summary of CaP axon outgrowth (hemisegments 8-12) in slg heterozygous, slg homozygous, splmo morphant and slg revertant embryos. For each group, a total of 25 axons from five embryos were scored as HMS, VNC, PVM or DVM. Error bars indicate s.e.m. of triplicate experiments. Lateral views, dorsal to the top and anterior to the left. Arrows indicate shortened CaP axons; arrowheads indicate abnormal branched axons. Scale bars: 20 μm in B-D′; 50 μm in E. PHENOTYPE:
|
|
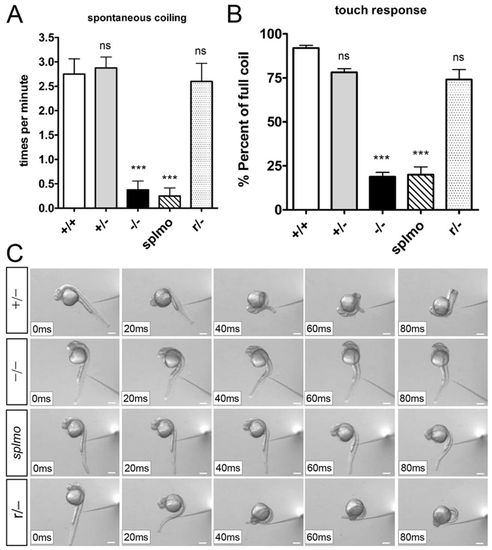
Reduction of Prdm14 expression causes embryonic movement defects. (A) Frequencies of spontaneous tail coiling in slg mutant and splmo morphant embryos are significantly reduced compared with wild-type and heterozygous embryos. The normal tail coiling is restored in revertant embryos. Ten embryos were examined in each group. Error bars indicate s.e.m. (B) The percentage of full tail coil in slg mutant and splmo morphant embryos after touch stimulus is significantly decreased compared with wild-type and heterozygous embryos. The normal movements were largely restored in revertant embryos. A total of 30-45 touch responses were monitored in each embryo and the percentage of full tail coil calculated. In each group, at least three embryos were recorded. Error bars indicate s.e.m. of triplicate experiments. ***P<0.001; ns, not significant; compared with wild type (two-tailed unpaired t-test). (C) Frame shots of typical twist movement in the touch response. See supplementary material Movies 1-5. Scale bars: 200 μm. PHENOTYPE:
|
|
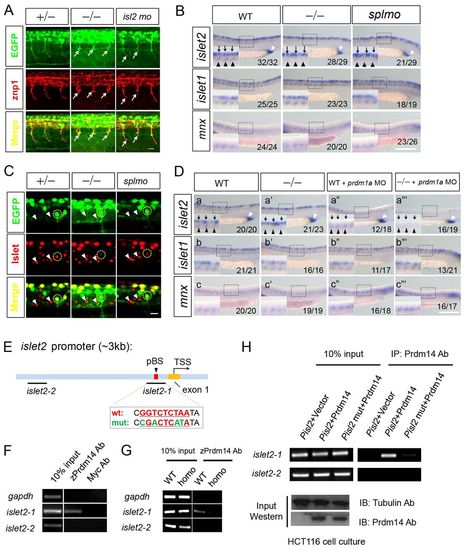
islet2 is a downstream target of Prdm14. (A) CaP axons are shortened in islet2 morphants and slg mutants. The embryos are stained with znp1 antibody. Arrows indicate shortened CaP axons. (B) islet2 RNA expression is only downregulated in CaP (black arrowheads) in slg mutant and splmo morphant embryos, whereas islet2 expression in RB (black arrows) and cloaca (white arrowhead) is unaffected. islet1 and mnx1 expression are unaffected by Prdm14 reduction. (C) Islet antibody staining indicates that the signal intensity of Islet2 (circled) in the CaP neurons is substantially reduced in slg mutants and splmo morphants compared with heterozygotes. Islet1 signal (arrowheads) in GFP-negative neurons (non-CaP) showed comparable staining intensity in embryos of all genotypes. (D) (a-a′′ ′) islet2 RNA expression is decreased specifically in CaP (black arrowheads) in slg mutants (a′). islet2 is specifically reduced in RB (black arrows) in prdm1a morphants (a3). prdm1a MO injection into slg mutants results in greatly decreased islet2 expression in both RB and CaP (a′′ ′). islet2 in cloaca remains unchanged (white arrowheads). (b-c3′) islet1 and mnx1 expression in RB and CaP remains largely unchanged when prdm14 and prdm1a are downregulated. (B,D) The number of embryos showing this phenotype out of the total examined is indicated bottom right. (E) The islet2 promoter contains a putative Prdm14 binding site (pBS). The conserved sequence among zebrafish, mouse and human is indicated in red; mutated nucleotides are in green. TSS, transcription start site. (F) ChIP-PCR shows that Prdm14 antibodies specifically precipitate an islet2 promoter fragment containing the pBS in wild-type embryos. (G) The ChIP signal from the Prdm14 antibodies is greatly decreased in slg mutants. (H) Zebrafish Prdm14 expressed in HCT116 cells efficiently pulls down the pBS-containing fragment when an islet2 promoter (Pisl2) plasmid is cotransfected. The PCR signal is greatly reduced when a pBS mutant (Pisl2 mut) plasmid is transfected or Prdm14 protein is not expressed in the cells. Scale bars: 20 μm in A,C; 200 μm in B,D. |
|
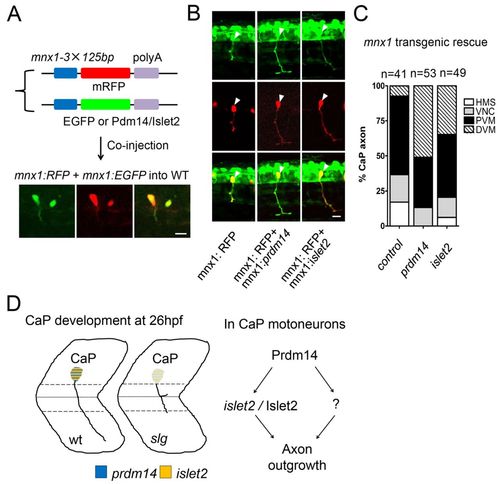
Transgenic islet2 expression in slg mutants rescues the axon outgrowth defect. (A) An mnx1-3×125bp enhancer sequence (mnx1) was used to direct protein co-expression in the same primary motoneurons (PMNs) from two injected plasmids (mRFP and GFP). (B) Expression of Prdm14 or Islet2 in CaP rescues the axon outgrowth defect of slg mutant zebrafish embryos. Arrowheads indicate CaP cell bodies. (C) Summary of results in B. n, number of RFP-labeled CaP neurons. n=41 from 20 injected embryos in the mnx:RFP group (control); n=53 from 33 injected embryos in the mnx:RFP + mnx:prdm14 group (prdm14); n=49 from 28 injected embryos in the mnx:RFP + mnx:islet2 group (islet2). (D) Proposed regulation of CaP axon outgrowth. Prdm14 regulates CaP axon outgrowth through islet2 and as yet unidentified factors (question mark). Scale bars: 20 μm. |
|
Expression patterns of the trapped gene in other neurons. (A-A′′) GFP expression in TGN and SAG in 36-hpf embryos. (B-B′′) Acetylated a-tubulin expression in TGN in 26-hpf embryos (circle, central axon; arrowheads, peripheral axons). (C-D′′) GFP expression in OSN, FBN and MBN in 26-hpf embryos. (E-E′′) Acetylated a-tubulin in OSN in 26-hpf embryos (square, OE; arrowheads, axons projected from OSN to OE). (F-F′′) GFP expression in MC, RSN and their afferents (MLF) in 3.5-dpf embryos. (G-G′′) Acetylated a-tubulin in RB in 26-hpf embryos. FBN, forebrain neurons; MBN, midbrain neurons; MC, Mauthner cells; MLF, medial longitudinal fasciculus; OB, olfactory bulb; OE, olfactory epithelium; OSN, olfactory sensory neurons; RSN, reticulospinal neurons; SAG, statoacoustic ganglion neurons; TGN, trigeminal neurons. Scale bars: 50 µm in A-D′′,F-F′&prime& 20 µm in E-E′′,G-G′′. EXPRESSION / LABELING:
|
|
Prdm14 is a nuclear protein and prdm14 is expressed before islet2 in zebrafish embryos. (A) Zebrafish Prdm14 is localized to the nucleus, similar to human PRDM14. The localization is ZF domain dependent. (B) The localization of different zebrafish Prdm14 isoforms is summarized. N, nucleus localized; C, cytoplasm localized. (C) prdm14 is expressed earlier than islet2 in CaP (arrows) at the 10-somite stage. Prdm14 downregulation causes decreased islet2 expression at the 20-somite stage. (D) Diagram illustrating the results in C. Scale bars: 10 µm in A; 200 µm in C. EXPRESSION / LABELING:
|