- Title
-
Regulation of a Vascular Plexus by gata4 Is Mediated in Zebrafish through the Chemokine sdf1a
- Authors
- Torregroza, I., Holtzinger, A., Mendelson, K., Liu, T.C., Hla, T., and Evans, T.
- Source
- Full text @ PLoS One
|
Hematopoiesis initiates normally in the gata4 morphant. Control (A, B) and gata4 morphant (C,D) embryos derived from the tg(gata1:dsred) reporter fish show that primitive erythropoiesis in the ICM is normal in gata4 morphants. A and C are brightfield; B and D are the fluorescent views. Views are lateral, anterior to the left. Control (E) and gata4 morphant (F) embryos stained for the macrophage marker l-plastin show that primitive myeloid development is also normal in the gata4 morphants. White arrows indicate blue-stained macrophages (M). Views are ventral, anterior to the top. For each, n >50. |
|
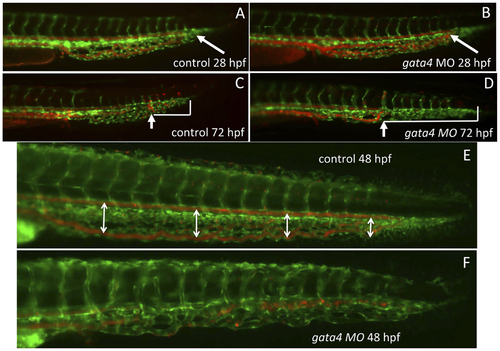
The vascular morphology of the CHT is disrupted in the gata4 morphant embryo. Shown are representative embryos (n >50) derived from tg(gata1:dsred; fli1:egfp) double transgenic reporter fish. The green signal shows the vascular endothelium, while the red signal shows the circulation of blood cells, which are moving so quickly that individual cells are not seen. At 28 hpf (A,B) the blood circulates to the caudal end of the developing CHT (the caudal end is indicated by white arrows) in both control uninjected (A) and gata4 morphant (B) embryos. However, by 72 hpf (C,D), the vascular plexus is much more developed in the control (C) compared to the morphant (D). In addition, the blood circulation is restricted in the morphant, failing to move into the more caudal regions, as indicated in panels C and D by the white brackets that mark the distance from the caudal end of the CHT to the most caudal position of circulating cells (indicated by the white arrows). The block to plexus formation can be seen clearly at 48 hpf (E,F), when in the control embryos (E) the dorsal aorta and caudal vein are well separated to create a fenestrated space (this space is indicated by double-headed arrows), which is not seen in the morphant (F). MO = morphant. Views are lateral, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
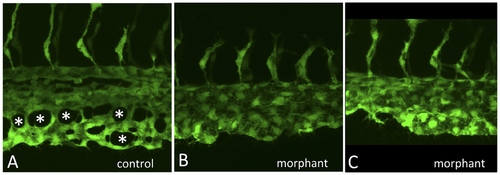
The gata4 morphant CHT fails to develop fenestrated vascular structures. Shown are higher resolution images of control (A) and two independent examples of morphant embryos (B,C) imaged at 32 hpf by confocal microscopy, as the caudal plexus is forming. While vasculogenesis initiates normally, and intersegmental vessels sprout equivalently, angiogenesis in the CHT of control embryos generates a vascular plexus containing open fenestrated structures, indicated by the asterisks in A, that are missing in gata4 morphants. |
|
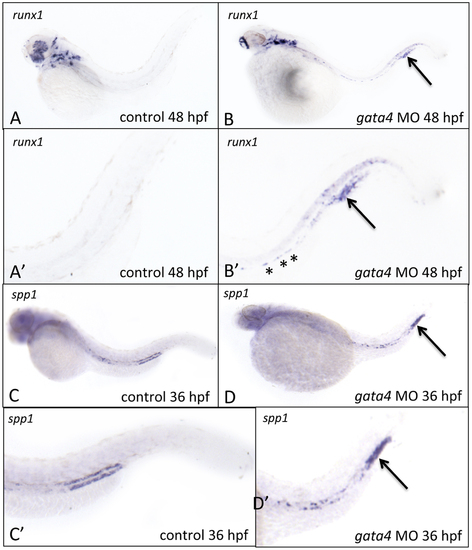
The CHT in gata4 morphants shows aberrant expression of runx1 and osteopontin. A-D: Shown are representative control (left panels) or gata4 morphant embryos following in situ hybridization to detect expression of runx1 at 48 hpf (A-B) or spp1 at 36 hpf (C-D). A′-D′ are higher magnification views of the tails from panels A-D, respectively. Asterisks in B′ indicate the presence of remaining runx1+ cells associated with the dorsal aorta in morphants. The strong expression in the tails of runx1 and spp1, indicated by the arrows, was reproducibly noted in 60% and 50% of gata4 morphants, respectively (n = 20 each per experiment). Views are lateral, anterior to the left. |
|
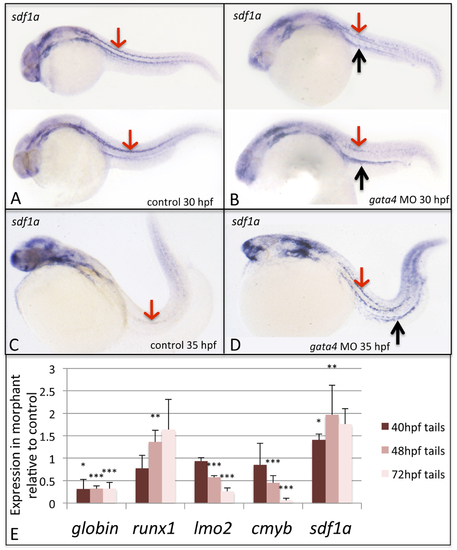
The sdf1a expression pattern is disturbed in gata4 morphants, while quantitative analysis confirms changes in expression in morphant embryos for sdf1a and hemato-vascular markers. Shown are representative embryos (n >50) following in situ hybridization to detect expression of sdf1a transcripts in control (A,C) and morphant (B,D) embryos, at 30 hpf (A,B) and 35 hpf (C,D). In panels A and B, 2 independent embryos are shown representing the range of patterns detected. Red arrows pointing down show the strong stripes of expression in lateral mesoderm in control embryos, which is nearly gone by 35 hpf. In contrast, the pattern is less clear in mesoderm of 30 hpf morphant embryos, but strongly persists by 35 hpf, and is much enhanced in the region associated with the pronephric tubules, particularly at 35 hpf (black arrows pointing up in panels B and D). Views are lateral, anterior to the left. (E) Tails including the CHT region were isolated from control and gata4 morphant embryos at 40, 48, or 72 hpf and processed by qPCR to measure relative levels of ae3globin, runx1, lmo2, cmyb, and sdf1a. The Y axis plots the relative transcript levels of morphants compared to controls (controls set at a value of 1). Each sample included >30 embryos, and data was collected in 3 independent experiments. Student’s T-test was used to determine significance (*p<0.05, **p<0.01, ***p<0.001). EXPRESSION / LABELING:
|
|
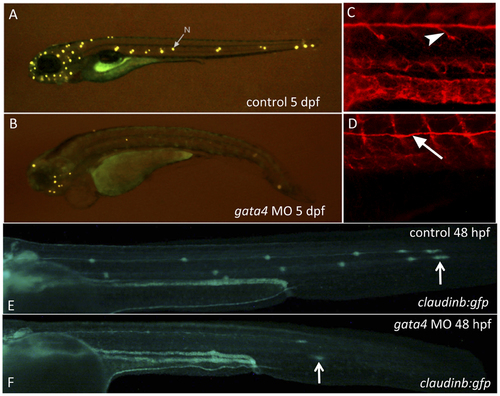
The gata4 morphants fail to develop neuromasts of the lateral line due to a failure in neuromast deposition. A–D: Shown are representative control (A) and gata4 morphant (B) embryos (n >50) that were stained at 5 dpf with 4-Di-2-Asp to detect differentiated neuromasts (the arrow in panel A points to a representative neuromast, N). Control (C) or morphant (D) embryos were stained with an anti-acetylated tubulin antibody to detect the neurons that migrate along with the primordium and innervate the neuromasts (arrowhead in C). While neurons fail to branch off in the morphants, the neuron tracks along the route of the primordium normally (arrow points to this neuron track in D). E–F: Shown are representative control (upper panel) and gata4 morphant (lower panel) embryos at 48 hpf (n >50) derived from the transgenic claudinb:gfp reporter line, which labels the primordium and the deposited neuromasts. Specific patches of deposited cells are present at bilateral positions along the lateral line in control embryos (white arrow in E indicates the leading edge of the migrating primordium on the left side of a control embryo). In morphant embryos the primordium always migrates, and often along the normal route of the lateral line. However, in morphant embryos the migration is delayed (the arrow in F indicates the “lagging” leading edge in a representative morphant embryo), and in some morphant embryos the route was abnormal (not shown). Views are lateral, anterior to the left. |
|
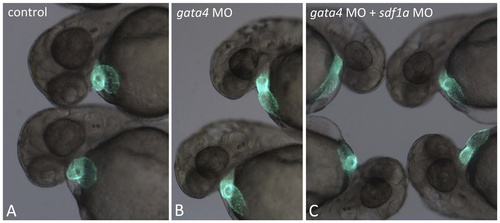
Depletion of sdf1a rescues the neuromast and circulation defects in the gata4 morphant. A: Shown are representative embryos at 72 hpf (n >50, multiple independent experiments) following incubation with 4-Di-2-Asp to detect neuromasts. For comparison with control embryos (left panels) embryos were injected with the gata4 morpholino and half the clutch was left alone (middle panels), while the other half was subsequently injected with the sdf1a morpholino (right panels). The lower panels are the fluorescence images of the corresponding brightfield panels. The examples shown are representative of the rescue for the lateral line in 34% of co-injected embryos (using 0.25 ng of the sdf1a morpholino). B: Shown are representative embryos at 72 hpf (n >50, multiple independent experiments) derived from the tg(gata1:dsRed) reporter line. For comparison with control embryos (left panels) embryos were injected with the gata4 morpholino and half the clutch was left alone (middle panels), while the other half was subsequently injected with the sdf1a morpholino (right panels). The lower panels are the fluorescence images of the corresponding brightfield panels. The rescue of circulation into the CHT plexus in the tail was observed in 82% of co-injected embryos (using 2 ng of the sdf1a morpholino). White arrowheads indicate the caudal vein in the CHT. Views are lateral, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
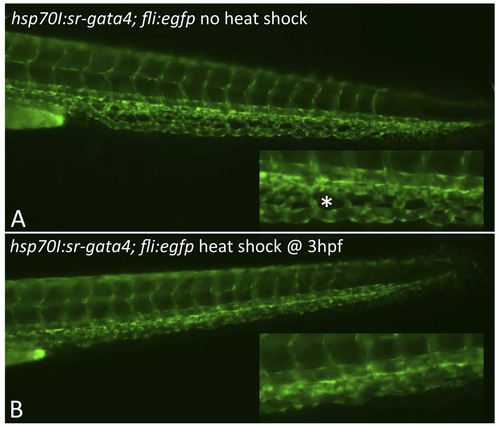
Expression of dominant-negative SR-Gata4 during gastrulation causes a block in development of the CHT plexus. Shown are representative transgenic embryos that were either left at normal temperature (A) or heat shocked for one hour at 37C starting at 3.5 hpf. Embryos are derived from crossing the tg(sr-gata4) line with the tg(fli:egfp) reporter strain. Those embryos carrying the transgene expressing SR-Gata4 are identified by RFP+ hearts (not shown here). Insets show magnified view of the equivalent regions of the developing CHT at 48 hpf. The normal fenestrated structures (an example indicated by the asterisk in A) were seen in 85% of the non-heat-shocked embryos, while in contrast, 84% of the heat-shocked embryos lacked these angiogenic structures. For each, n>50. |
|
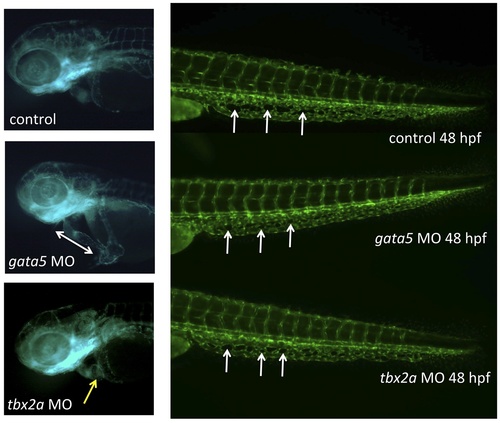
The CHT defect caused by depletion of Gata4 is not seen in other models of embryonic cardiomyopathy. The heart and caudal tail regions are shown in representative 48 hpf tg(gata1:dsred; fli1:egfp) uninjected control embryo (top), or embryos that had been injected with morpholinos targeting gata5 (middle) or tbx2a (bottom). In the trunk images, white arrows indicate examples of characteristic fenestrated structures that start to form around 32 hpf in the CHT plexus, but fail to do so in the gata4 morphant. The control embryo has a normally looped heart, while the gata5 morphant has an unlooped heart-string (indicated by the double-headed arrow), and the tbx2a morphant has an extended dysmorphic atrium (indicated by the yellow arrow). Morpholinos were used under conditions that generate reproducible cardiomyopathies and poor circulation with pericardial edema, as documented in our own and others studies: gata5, 52-AAGATAAAGCCAGGCTCGAATACAT, 10 ng/embryo; tbx2a,52-CGGTGCATCCAACAAACGTAGTGAA, 5 ng/embryo. |
|
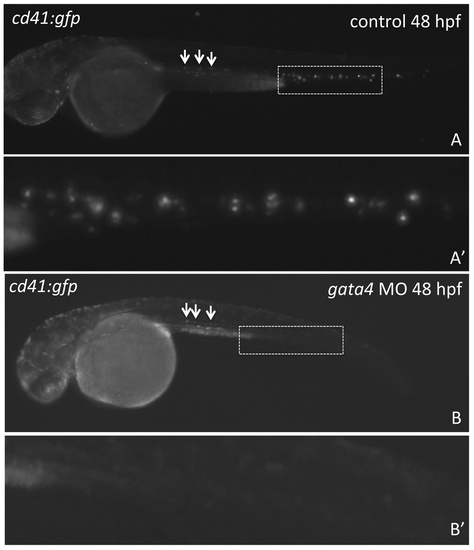
Progenitors that develop in association with the dorsal aorta fail to populate the CHT. Shown are representative embryos (n >50) derived from tg(cd41:gfp) transgenic reporter fish, in which GFP expression marks the wave of definitive progenitors that are born in the floor of the dorsal aorta (indicated by the arrows in A, B). At least some of these migrate and seed the CHT of control embryos (boxed area in A). While the appearance of GFP+ cells from the dorsal aorta appears normal in the gata4 morphant embryos (arrows in B), GFP+ cells are never found in the morphant CHT (equivalent boxed area in B). A′ and B′ are enlarged views of the dashed boxes shown in A and B. Views are lateral, anterior to the left. |
|
Modulation of Sdf1a is not sufficient to rescue the cardiac defect of gata4 morphants. A) Control uninjected embryos at 2 dpf have normal looped hearts, as visualized in the tg(myl7:gfp) transgenic reporter background. B) Hearts fail to loop in the gata4 morphant embryos. C) Likewise, the hearts fail to loop properly in gata4 morphants that are co-injected with the sdf1a morpholino. In the example shown here, 2 ng of sdf1a morhoplino was used, but we also failed to reproducibly rescue cardiogenesis with higher or lower doses. |
|
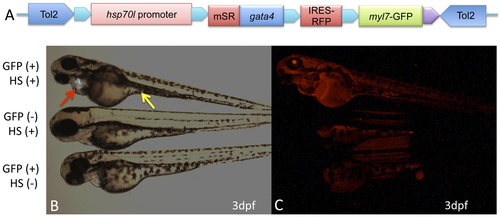
Conditional expression of SR-Gata4 phenocopies the gata4 morphant. A) The schematic shows the transgenic construct used to generate lines of tg(hsp70I:sr-gata4) zebrafish. In the context of a tol2-flanked destination vector, the hsp70I promoter was used to direct expression of SR-Gata4, followed by an IRES that allows co-expression of RFP (cherry). An myl7 promoter directing expression of GFP to the heart is also present as a marker for transgenesis. B) Shown are representative examples of three cohorts of embryos obtained by crossing zebrafish heterozygous for the transgene, imaged under fluorescence with the green channel. The top embryo is GFP+, indicating that the embryo carries the transgene. This embryo was also heat-shocked at 37C for one hour, in this case at 5, 24, and 27 hpf. The embryo in the middle was treated the same, but is GFP-, indicating that this sibling does not carry the transgene. The bottom embryo is GFP+ (transgenic) but was kept at 28.5C (no heat shock, HS-). Note that only the top embryo shows the characteristic gata4 morphant phenotype: an improperly looped heart and pericardial edema (red arrow) and a failure in the yolk stalk extension (yellow arrow). C) The same embryos were imaged under the red channel, showing that only the top embryo expresses RFP, since it is the only embryo that is both transgenic and heat shocked. Essentially every transgenic embryo shows this heat-shock dependent phenotype (n>100). |