- Title
-
Targeted deletion of the zebrafish obscurin A RhoGEF domain affects heart, skeletal muscle and brain development
- Authors
- Raeker, M.O., Bieniek, A.N., Ryan, A.S., Tsai, H.J., Zahn, K.M., and Russell, M.W.
- Source
- Full text @ Dev. Biol.
|
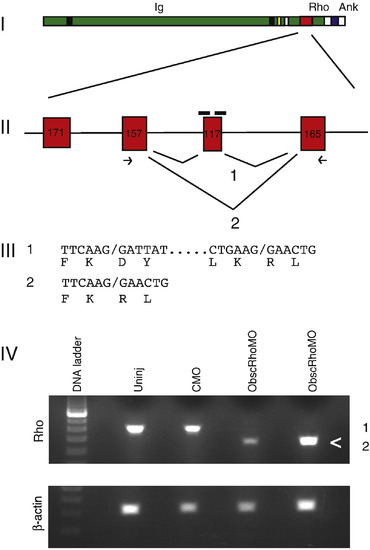
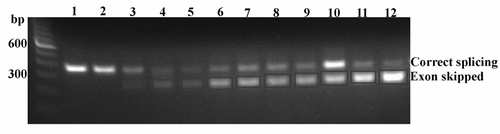
Targeting of the obscurin A RhoGEF domain. (I) Diagram of obscurin A demonstrating the location of the tandem immunoglobulin-like (Ig) domains, the RhoGEF domain (Rho) and the ankyrin binding (Ank) domains. (II) Diagram of the exons encoding the double homology (DH) domain of the RhoGEF module. A 117-bp exon encoding for part of the GTPase activation site of the RhoGEF domain was targeted for induced exon skipping by the injection of antisense morpholinos (black bars) directed at the splice donor and acceptor sites for that exon. (III) Splice product 1 represents the normal splicing. The morpholinos are frequently able to block normal splicing of the targeted exon resulting in the splice product 2. PCR primers (arrows) from the adjacent exons were used to detect the splice products. Both products were cloned and sequenced and the nucleotide and predicted amino acid sequences displayed. (IV) RNA from 48 hpf embryos was isolated from obscurin A RhoGEF (ObscRhoMO) morphant, uninjected (Uninj) control morpholino-injected (CMO) embryos. Note that the band (<) corresponding to splice product 2 is only present in the RNA isolated from the morphant embryos. |
|
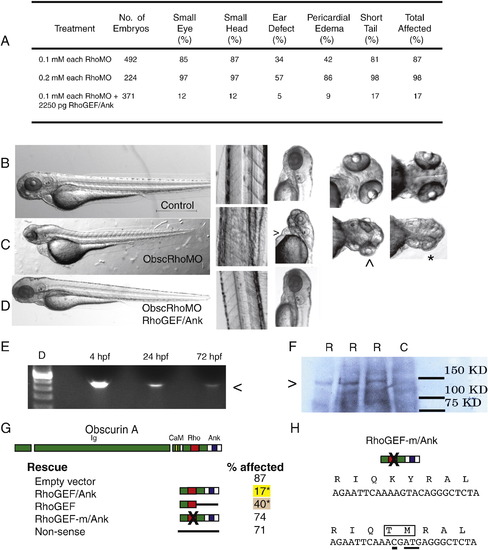
Obscurin A RhoGEF morphant phenotype. (A) Morphant embryos were assessed at 72 hpf and scored for head size (small head), eye morphology (small eye, poorly developed optic cup), ear morphology (one or no otoliths), cardiac structure and function (ventricular hypoplasia or dilatation, pericardial edema) and skeletal muscle appearance (reduced tail length and width). (B–D) Gross morphology of control (B), obscurin A RhoGEF morphant (C) (0.1 mM of each morpholino), and rescued obscurin A RhoGEF morphant embryos (D) (0.1 mM of each morpholino + 2250 pg RhoGEF/Ank expression construct). The obscurin A RhoGEF morphant embryos demonstrate a high incidence of pericardial edema (>), moderate (^) to severe (∗) eye hypoplasia, and smaller heads than controls. These defects were rescued or markedly diminished by co-injection of an obscurin A expression construct that included the RhoGEF and ankyrin binding domains (RhoGEF/Ank). (E) RT-PCR of mRNA extracted from 4, 24 and 72 hpf embryos injected with the expression construct demonstrating correct amplification of the expression tag (<). (F) Western analysis (Ab: anti-EGFP) of 72 hpf whole embryo lysates demonstrating expression of a fusion protein (>) of the expected size only in injected embryos (R) and not in control (C). (G) Percentage of obscurin A RhoGEF morphant embryos demonstrating one or more of the phenotypic abnormalities upon co-injection with the indicated rescue construct (∗p < 0.05 compared to empty vector control). Note the high efficiency of rescue with the RhoGEF/Ank and the absence of rescue with the non-sense and RhoGEF-m/Ank constructs. (H) Diagram of the amino acid sequence change introduced into the RhoGEF-m/Ank construct that replaces a conserved KY (Lysine–Tyrosine) motif with TM (Threonine–Methionine). Underlined nucleotides were changed using site-directed mutagenesis. PHENOTYPE:
|
|
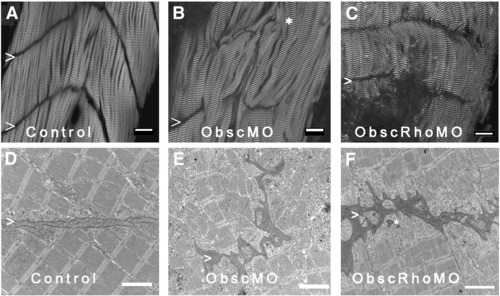
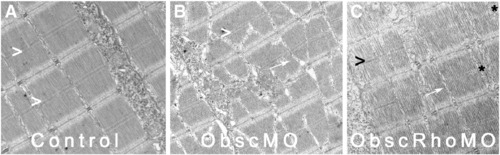
Architecture of the myotendinous junction (MTJ). Confocal analysis using α-actinin (a Z disk component) immunolabeling (A–C) to assess myofibril morphology and ultrastructural analysis (D–F) to define the architecture of the MTJ was performed in control (A, D), obscurin A morphant (B, E) and obscurin A RhoGEF morphant (C, F) embryos at 72 hpf. In control embryos, the skeletal myocytes are aligned in parallel and the myotendinous junctions are well defined (A, D: >). The skeletal myocytes in obscurin A (ObscMO) morphant embryos vary in length and in their relationship to each other (∗) with elongated myocytes extending past very rudimentary somite boundaries (B, E: >). When compared to the ObscMO embryos, the obscurin A RhoGEF (ObscRhoMO) morphant embryos demonstrate a more consistent relationship between adjacent myocyte (more uniform parallel arrangement) and normal positioning of a complete or nearly complete somite boundary (C,F: >). However, the boundary is not sharply defined as in control embryos and often appears electron dense and irregular, not unlike the rudimentary boundaries/MTJs noted in ObscMO embryos. Scale bars are 20 μm (A–C) and 2 μm (D–F). PHENOTYPE:
|
|
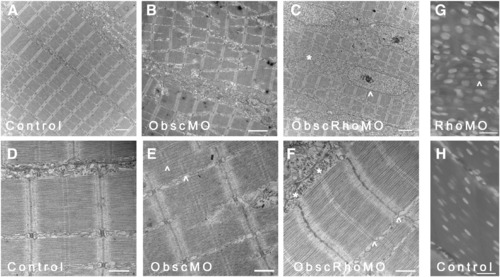
Skeletal muscle phenotype in obscurin A RhoGEF morphant embryos at 72 hpf. Ultrastructural analysis was performed at 72 hpf on control, obscurin A (ObscMO) and obscurin A RhoGEF (ObscRhoMO) morphant embryos. In control embryos, the myofibrils are aligned in register across the sarcoplasm (A) with well defined M bands and finely “stitched” Z disks (D). In the obscurin morphant embryos (B) there is marked disturbance of myofibril organization with areas of misalignment of the M bands of adjacent myofibrils (E: ^). By comparison, the obscurin A RhoGEF morphants display myofibril organization (C) similar to control embryos but reduced myofibrillar content with more sarcoplasm devoid of myofibrils and more rounded nuclei (C, G: ^). There were also mild sarcomeric abnormalities including, infrequently, an indistinct appearance of the Z disk and absence of the M band (F: ∗) which could occur even within myofibrils demonstrating mature Z disks and M bands (F: ^). Note that in skeletal myocytes stained with DAPI, the nuclei in the obscurin A RhoGEF morphants (RhoMO) are closer together and more rounded (G) than in controls (H) consistent with reduced myofibril volume. Scale bars 2 μm (A–C), 0.5 μm (D–F) and 10 μm (G, H). PHENOTYPE:
|
|
Absence of consistent M bands in obscurin A RhoGEF skeletal muscle at 72 hpf. Control embryos (A) consistently demonstrate well-formed M bands (>) in skeletal myofibrils by 72 hpf. Obscurin A morphant embryos (B), although they display marked abnormalities in myofibrillar architecture, also consistently demonstrate mature M bands (>). In contrast, skeletal myofibrils in obscurin A RhoGEF morphant embryos (C) demonstrated occasional absence of M bands (∗) in otherwise mature-appearing myofibrils. Note that the extrajunctional SR in the ObscMO embryo is markedly disorganized (B: arrow), but is much more normal appearing in the ObscRhoMO embryos (C: arrow). PHENOTYPE:
|
|
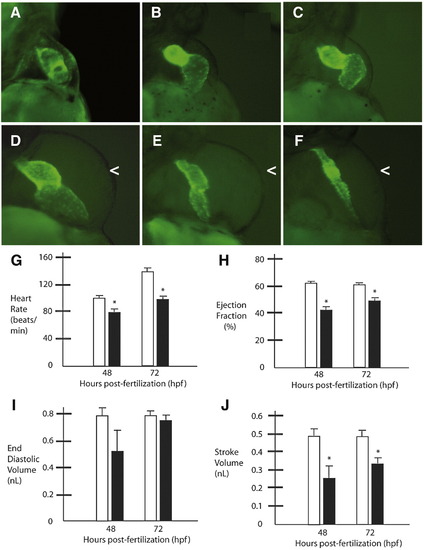
Obscurin A RhoGEF signaling is required for normal cardiac development. Cmlc2-EGFP transgenic zebrafish embryos injected with obscurin A RhoGEF morpholinos (B–F) were compared to control-injected transgenic embryos (A) at 72 hpf. Morphant embryos demonstrated a spectrum of cardiac abnormalities ranging from reduced cardiac function with cardiac dilatation and mild pericardial edema (B–D) to mild (E) or severe (F) ventricular hypoplasia and pericardial edema (<). (G–J) Cardiac functional assessments were performed in morphant (black bars) and control embryos (white bars) at 48 and 72 hpf. In the more mildly affected embryos with mild ventricular hypoplasia as determined by comparable end diastolic volume measurements relative to controls (I), there were significant reductions in heart rate (G), ejection fraction (H) and stroke volume (J) at 48 and 72 hpf compared to controls (∗t-test; p < 0.05). PHENOTYPE:
|
|
Ultrastructural analysis of cardiac myocytes at 72 hpf. (A) Cardiac myocytes in control embryos have formed intercalated disks (^) and filled the sarcoplasm with mature myofibrils. (B) Higher magnification of an intercalated disk (ID) from a control embryo demonstrates adherens (>) and gap (^) junctions. (C, D) In contrast, cardiac myocytes from mildly affected obscurin A RhoGEF morphant embryos have reduced myofibrillar content [compare the nuclear (N) area to the myofibrillar area within each myocyte] and very poorly defined regions of cell–cell (^) contact with no identifiable IDs. Examples of recent nuclear division (N+) were more frequent in the obscurin RhoGEF morphant embryos. (E, F) In more severely affected embryos, cardiac myocytes appear markedly abnormal with loosely arranged contractile filaments (F: ∗). (G, H) The mature cardiac myofibrils that did form in the obscurin A RhoGEF morphant embryos were not significantly different than control with a normal appearing lattice of thick filaments on cross-section (G) and laterally aligned Z disks and M bands on longitudinal section (H). Scale bars are 4 μm (A–C), 2 μm (D, E), 1 μm (F) and 0.5 μm (G, H). PHENOTYPE:
|
|
Eye phenotype of obscurin A RhoGEF embryos. Control (A, B, E) and obscurin RhoGEF (C, D) morphant embryos were immunolabeled for Obscurin (A,E) or RhoA (B–D) at 72 hpf. In the control embryo, distinct retinal layers are noted [ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer (ONL)]. Obscurin is expressed in each of the layers, usually at the cell periphery with the greatest abundance in the axon-rich IPL (A) and at the apical and basal aspects of the ONL (E) in a distribution similar to RhoA (A, B). In the mildly affected obscurin A RhoGEF morphant embryos (C), rudimentary GCL, IPL, INL, OPL, and ONL are noted while the more severely affected embryos do not develop identifiable layers (D). On ultrastructural analysis, there is significant pigment deposition and a well developed photoreceptor layer (^) in the retina of a control embryo (F) while even a mildly affected morphant embryo (G) has very little pigmentation and no identifiable photoreceptors. A more severely affected embryo (H) lacks even rudimentary layers and the cells remain elongated and poorly differentiated. Scale bars are 20 μm (A–D) and 10 μm (E–H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
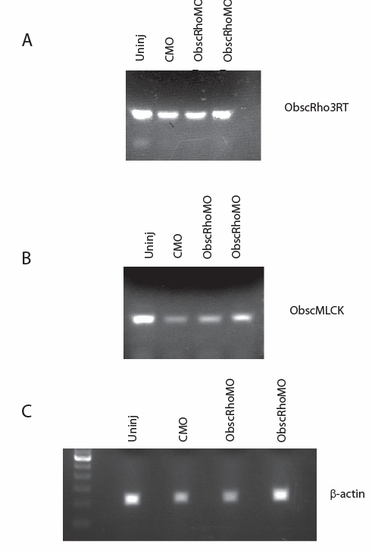
RT-PCR of analysis of RNA isolated from 48 hpf uninjected (Uninj), control morpholino-injected (CMO) and obscurin RhoGEF morpholino-injected (ObscRhoMO) embryos. (A) Amplification using primers for the 3′ end of the obscurin A transcript demonstrates intact expression of the transcript 3′ to the targeted exon. (B) Amplification of the obscurin B transcript indicates that the transcript is intact but not appreciable upregulated in response to obscurin A RhoGEF antisense targeting. (C) β-actin amplification is shown for comparison. |
|
1- 0.2mM Control; 2- 0.4 mM Control; 3- 0.2 mM Rho Ex-Int MO; 4- 0.4 mM Rho Ex-Int MO; 5, 6, 7, 8- 0.2 mM Rho Int-Ex MO; 9 and 10- 0.4 mM Rho Int-Ex MO; 11- 0.1 mM Rho Ex-Int MO + 0.1 mM Rho Int-Ex MO; 12- 0.2 mM Rho Ex-Int MO + 0.2 mM Rho Int-Ex MO. |
|
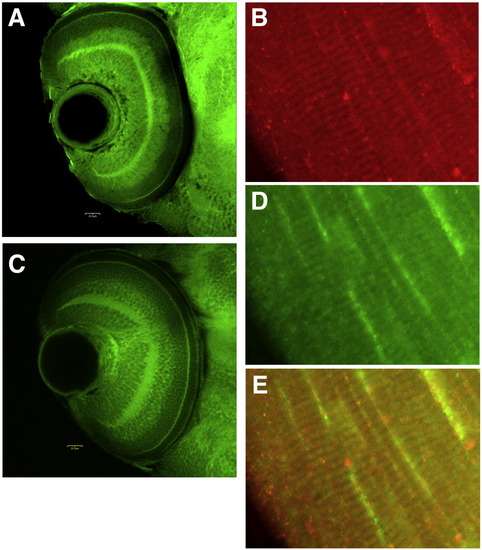
Expression of obscurin (A, B) and RhoA (C, D) in retina (A, C) and skeletal muscle (B, D) at 72 hpf. E shows the overlay of muscle expression. |
Reprinted from Developmental Biology, 337(2), Raeker, M.O., Bieniek, A.N., Ryan, A.S., Tsai, H.J., Zahn, K.M., and Russell, M.W., Targeted deletion of the zebrafish obscurin A RhoGEF domain affects heart, skeletal muscle and brain development, 432-443, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.