- Title
-
A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression
- Authors
- Posner, M., Hawke, M., Lacava, C., Prince, C.J., Bellanco, N.R., and Corbin, R.W.
- Source
|
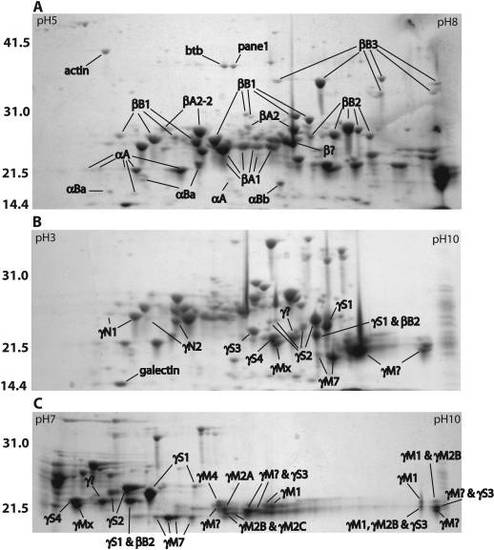
Two-dimensional electrophoresis (2-DE) profiles of total adult zebrafish lens protein. Separation was performed on 11 cm IPG strips with pH gradients of 5–8 (A), 3–10 nonlinear (B) and 7–10 (C). Gels were Coomassie stained and spots were identified by MALDI-TOF MS. Mass standards are indicated on the left in kiloDaltons. Spots labeled with a “?” contain two or more crystallins from the indicated family. Accession numbers for each crystallin are shown in Table 1. The accession numbers for four identified non-crystallins are as follows: actin (AAO38846), BTB (POZ) domain containing protein 2 (NP_001038557), proliferation associated nuclear panel 1 (Pane1; NP_991153), and galectin-related inter-fiber protein (XP_684452). EXPRESSION / LABELING:
|
|
Phosphoprotein staining of two-dimensional electrophoresis gels (pH 5–8) indicates phosphorylated αA-crystallins. Numbers identify the equivalent αA-crystallin spots on gels stained with the total protein stain (A) and the phosphoprotein-specific stain (B). Labels and arrows indicate α-crystallin spots that were not detected by the phosphoprotein stain. EXPRESSION / LABELING:
|
|
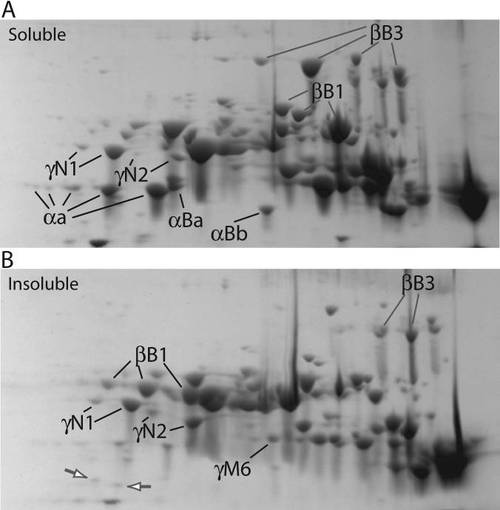
Comparison of soluble and insoluble protein from adult zebrafish lens. Both soluble (A) and insoluble (B) fractions were focused on pH 5–8 IPG strips before SDS–PAGE. Labels indicate examples of spots that are more abundant in one specific protein fraction (α-, βB1-, βB3-, and γN2-crystallin) or are equal in abundance in both fractions (γN1-crystallin). The white arrows on gel B show two α-crystallins that are preferentially found in the insoluble fraction. Many of the preferentially insoluble spots appear to be truncations. |