- Title
-
Zebrafish colgate/hdac1 functions in the non-canonical Wnt pathway during axial extension and in Wnt-independent branchiomotor neuron migration
- Authors
- Nambiar, R.M., Ignatius, M.S., and Henion, P.D.
- Source
- Full text @ Mech. Dev.
|
col mutant embryos display a late axial extension phenotype. Live col mutants at 4 dpf (B) have wider notochords (n) than their wildtype siblings (A). Larger notochord diameter is observed in cross-sections of the trunk of 2 col mutants stained with antibody f59 (D) as compared to wildtype (C). col mutants also display rounded somite morphology (B) unlike chevron-shaped wildtype somites (A). In contrast, col mutants do not display prominent early CE defects. For example, ntl expression in the notochord anlage at late gastrulation of col embryos (F) is indistinguishable from wildtype embryos (E). The degree of convergence of the neural plate border at the 12-somite stage identified by foxd3 expression also appears normal in col mutants (G and H). EXPRESSION / LABELING:
|
|
Facial (nVII) hindbrain neurons in col mutants do not migrate tangentially. Hindbrain neurons are marked by islet1 expression (A and B) at 56 hpf. In col mutant, nVII neurons fail to migrate into r6 and r7 (B) as in wildtype siblings (A). In contrast, nV neurons are positioned correctly in mutants. |
|
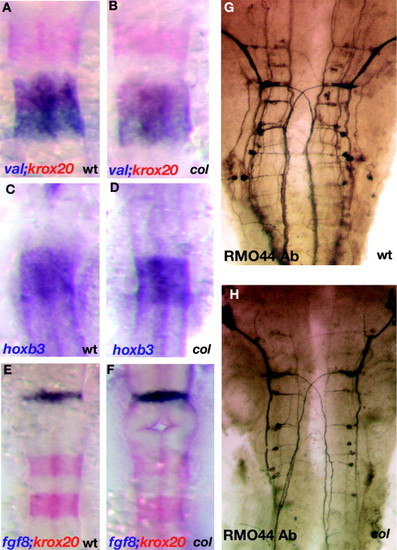
Gross patterning of the hindbrain is unperturbed in col mutants. Hindbrain rhombomere patterning appears undisturbed in col mutant embryos (B, D and F) as compared to wildtype (A, C and E). Krox20 (red) labels r3 and r5 and valentino (blue) marks r5 and r6 (A and B). hoxb3 also marks r5 and r6 (C and D). fgf8 (blue) labels the mid-hindbrain boundary and krox20 (red) marks r3 and r5 showing that gross development between the mid-hindbrain boundary and r3 in the hindbrain also appears normal in col mutant embryos (F as compared to E). RMO44 labels reticulospinal neurons (G and H). The patterning of this neuronal population is relatively unperturbed in col mutants although overall neuronal numbers appear to be reduced (see Section 2). EXPRESSION / LABELING:
|
|
Regulators of the Wnt/PCP pathway are able to partially rescue col mutants. ΔNdsh (dsh; B and F) and rok2 (C and E) were able to abolish the short body axis (B and C) and short wide notochords (E and F) observed in col mutants at 3 dpf (A and D). However, canonical Wnt signaling regulated defects in col mutants still remain in injected embryos. dlx2 expression in the telencephalon (asterisk) remains reduced compared to wildtype (G) in rock2 injected col mutant embryos (I) as compared to uninjected col mutants (H). The persistence of this phenotype is also observed in ΔNdsh injected col mutants (not shown). EXPRESSION / LABELING:
|
|
tri/vangl2 is able to rescue extension defects and tangential migration of hindbrain branchiomotor neurons in col mutant embryos. Overexpression of tri/vangl2 in col mutants (B and D) is able to rescue the stunted trunk development and broad notochords observed in uninjected col mutants (A and C). Facial (nVII) hindbrain motor neurons labeled by islet1 expression are positioned ectopically in r4 in col mutants at 56 hpf (F) compared to wildtype (E). Overexpression of tri/vangl2 restores tangential migration of these neurons into r6 and r7 (G) as in wildtype siblings (E). nV neurons are positioned correctly in r2 in tri/vangl2 injected (G) and uninjected (F) col mutants. |
|
The col locus encodes hdac1. col maps close to the zebrafish hdac1 locus on LG 19 (A). A schematic showing the genomic organization of the zebrafish hdac1 gene (B). The zebrafish hdac1 gene has 15 exons and 14 introns spanning 9.94 kb of the genome and a T–G transversion in the intronic sequence adjacent to exon 5 causes aberrant splicing between exons 4 and 5 in col mutants (C). Aberrantly spliced hdac1 cDNA fragment in col with an additional 9 bp running in lane 1 can be distinguished from the wildtype product running in lane 2 (D). hdac1 expression is unaffected in 2 dpf col mutants (F, H) compared to wildtype (E and G). |
|
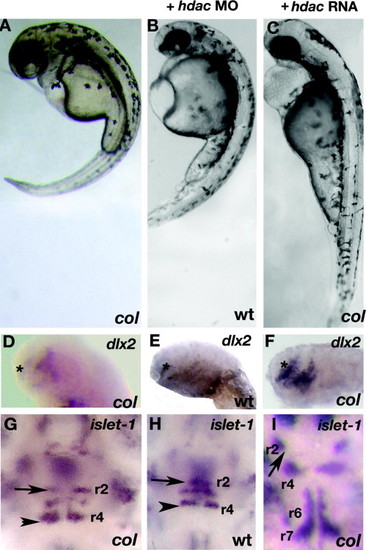
hdac1 morphants phenocopy col mutants and hdac1 RNA rescues col mutant phenotypes. hdac1 MO (hdac MO) injected wildtype embryos at 3 dpf (B) resemble uninjected col mutants (A). Reduced dlx2 expression (asterisk) and ectopically positioned facial hindbrain motor neurons (black arrowheads) in col mutants (D and G) are phenocopied in hdac1 morphants (E and H). The position of nV neurons in r2 are marked with arrows (G–I). hdac1 RNA (hdac RNA) is able to rescue axis extension and melanophore defects in col mutants (C). Telencephalon dlx2 expression and migration of facial hindbrain motorneurons is restored in col mutants injected with hdac1 RNA (F and I compared to D and G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
CE defects in early TSA-treated wildtype embryos resembles other zebrafish Wnt/PCP mutants. foxd3 expression at the 6-somite stage in TSA-treated embryos shows defects in convergence (arrowheads; B) as compared to wildtype (A). ntl expression at the 6-somite stage (C and D) and 22 hpf (E and F) reveals shorter, thicker notochord in TSA-treated embryos (D and F) than in wildtype embryos (C and E). myoD expression at 22 hpf shows compressed, laterally expanded somites in TSA-treated embryos (H) as compared to wildtype (G). EXPRESSION / LABELING:
|
|
Wildtype embryos treated late with TSA phenocopy col mutants. Live wildtype embryos at 2 dpf treated with 800 nM TSA late (at 16 hpf) resemble col mutants (A, compare to Fig. 7A). dlx2 expression is unaffected in these embryos (C; *) while the facial hindbrain motorneurons remain in r4 (arrowhead; E) as in col mutants. The position of nV neurons are shown with black arrows. Phenotypes observed at 2 dpf in wildtype embryos treated with 50 nM TSA early (5 hpf) appear more severe (B). The forebrain dlx2 expression domain is reduced (D; *), similar to col mutants, and facial hindbrain motorneurons remain in r4 (F) in these embryos. EXPRESSION / LABELING:
|
|
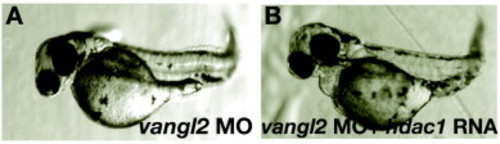
hdac1 RNA does not rescue defects in vangl2 morphants. vangl2 morphants injected with hdac1 RNA (B) still display axial extension defects observed in vangl2 morphants (A). The tangential migration defect of hindbrain nV11 branchiomotor neurons in vangl2 morphants is also not rescued by hdac1 misexpression (not shown). PHENOTYPE:
|
|
Overexpression of hdac1 in wildtype embryos causes brain and trunk defects. Injection of hdac1 RNA causes and enlargement of the forebrain (arrows) at moderate doses (B, compare to A). At high concentration hdac1 RNA causes loss of forebrain tissue and an expansion of midbrain (D, compare to C) accompanying severe loss of trunk tissues. wnt1 expression marks the posterior edge of the midbrain (arrow E) in uninjected wildtype embryos at 2 dpf. Embryos injected with hdac1 RNA display an antero-posterior expansion of wnt1 expression (arrows). |
|
Statistical analysis of mean length of wildtype and col/hdac1 embryos at 25, 48 and 72 hpf PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Mechanisms of Development, 124(9-10), Nambiar, R.M., Ignatius, M.S., and Henion, P.D., Zebrafish colgate/hdac1 functions in the non-canonical Wnt pathway during axial extension and in Wnt-independent branchiomotor neuron migration, 682-698, Copyright (2007) with permission from Elsevier. Full text @ Mech. Dev.