- Title
-
Specification of epibranchial placodes in zebrafish
- Authors
- Nechiporuk, A., Linbo, T., Poss, K.D., and Raible, D.W.
- Source
- Full text @ Development
|
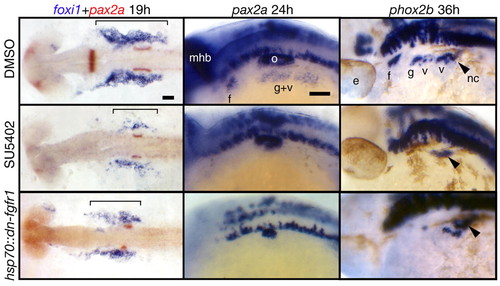
Fgf signaling is required for EB placode development. hsp70::dn-fgfr1 embryos (bottom panels) were heat shocked at 10 hpf (bud stage), collected at 19, 24 and 36 hpf and processed for in-situ hybridization with foxi1, pax2a and phox2b, respectively. Top and middle panels show embryos treated with the DMSO and the Fgfr inhibitor SU5402, respectively, beginning at 10 hpf and processed in the same way as the hsp70::dn-fgfr1 embryos. All panels are lateral views, except foxi1+pax2a panels that show dorsal views. Anterior is at left. As expected, pax2a expression in the midhindbrain boundary and the otic vesicle was either absent or strongly reduced in the Fgf-depleted embryos. In the SU5402-treated and hsp70::dn-fgfr1 embryos, foxi1 expression is strongly reduced (bracket), and pax2a expression in the EB placodes is absent. phox2b was not expressed in EB placodes in SU5402-treated embryos; its expression was strongly reduced in hsp70::dn-fgfr1 embryos, but a few phox2b-positive neurons remain in the large vagal ganglion. We presume that this milder phenotype resulted from the degradation of the transgenic protein. Black arrowheads mark the phox2b+ vagal neural crest cells (not affected in Fgf-depleted embryos) on their ventral migration route just posterior to the last branchial arch. These cells can be easily identified, because they make a characteristic turn toward the gut (Shepherd et al., 2004). Scale bars: 50 μm. e, eye; f, facial placode or ganglion; g, glossopharyngeal placode or ganglion; mhb, midhindbrain boundary; nc, vagal neural crest; o, otic vesicle; v, vagal placode or ganglion. EXPRESSION / LABELING:
PHENOTYPE:
|
|
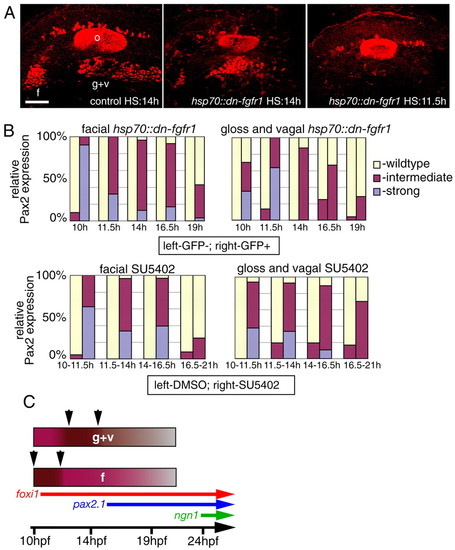
Temporal requirement for FGF signaling. (A,B) Wild-type embryos were incubated for 1.5-4.5 hours in SU5402 starting at 10, 11.5, 14 and 16.5 hpf (DMSO-treated embryos were used as controls); heterozygous hsp70::dn-fgfr1 fish were crossed to wild type and their progeny was heat shocked at the same time points (GFP-negative embryos were used as controls). In both cases, resulting embryos (6-14 per each time point) were analyzed using Pax2 antibody at 24 hpf and scored for either presence (A, left), reduction (A, middle), or absence (A, right) of the facial (B, left) and glossopharyngeal and vagal (B, right) placodes. (C) Summary of the data in (B). Note that Fgf signaling is strongly required between 10 and 11.5 hpf in the facial placode (f, arrows) and between 11.5 and 14 hpf in the glossopharyngeal and vagal placodes (g+v, arrows), implying that the EB placodes are formed in a rostral to caudal sequence. Scale bars: 50 μm. Abbreviations are as in Fig. 1. |
|
Fgf signaling is required cell autonomously in EB placodes. (A) hsp70::dn-fgfr1 donor embryos were injected with a lineage tracer (fluorescein-dextran, green). At shield stage, 25-30 donor cells were transplanted into the prospective placodal domain of wild-type hosts. Mosaic embryos were heat shocked at 10-11 hpf, collected at 24 hpf and analyzed for Pax2 protein expression (red). Panels show side view of the embryos that received either wild-type (A, top) or hsp70::dn-fgfr1 cells (A, middle and bottom). Wild-type cells readily contributed to the EB placodes (arrowheads), whereas hsp70::dn-fgfr1 cells either accumulated dorsally (arrows) or were excluded from EB placodes (A, bottom). Dotted line indicates a patch of hsp70::dn-fgfr1 cells within the EB placode that did not express Pax2. (B) In reciprocal experiments, wild-type donor embryos were injected with a lineage tracer (fluorescein-dextran, green). At shield stage, 25-30 donor cells were transplanted into the prospective placodal domain of hsp70::dn-fgfr1 hosts, mosaic embryos were heat shocked at 13.5-16 hpf, collected at 24 hpf and analyzed for Pax2 protein expression (red). Because dn-Fgfr1 protein is localized to the membrane, while fluorescein is evenly distributed throughout the cell, wild-type donor cells were easily distinguishable in the transgenic host embryos. Most of the GFP in the host is not visible (except hindbrain), because the image brightness and contrast were adjusted to visualize much brighter fluorescein-positive donor cells. Transplanted wild-type cells contribute to EB placodes in hsp70::dn-fgfr1 embryos (arrowheads, top). Scale bars: 50 μm. o, otic placode. |
|
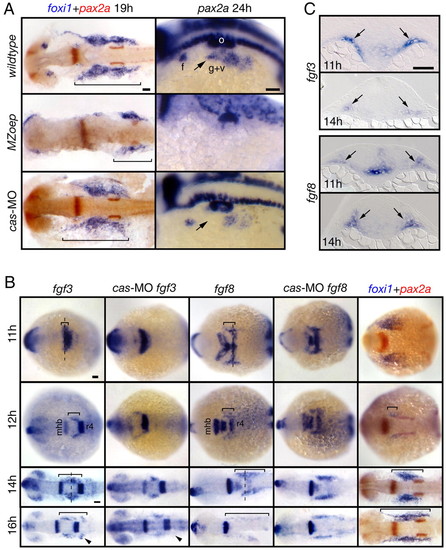
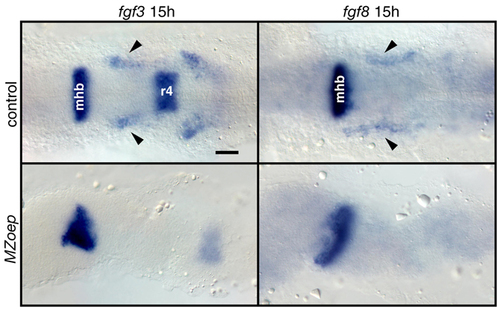
Mesoderm is the likely source of the EB placode-inducing signal(s). (A) MZoep mutants and cas morphants were analyzed for foxi1 and pax2a expression. foxi1 panels show dorsal views, and pax2a panels show lateral views. pax2a expression was absent and foxi1 expression was strongly reduced in MZoep embryos (bracket). In contrast, in cas morphants, foxi1 expression was normal and pax2a expression was slightly reduced (arrow). (B) Wild-type and morphant embryos were collected at 11, 12, 14 and 16 hpf and analyzed for fgf3, fgf8 and foxi1 expression. All panels show dorsal views. The presumptive mesodermal expression of fgf3 and fgf8 is marked by a bracket. Note that these expression domains expand first rostrally and then caudally with time and the expression pattern is largely unchanged in cas morphants, with the exception of a small fgf3-expressing domain (arrowheads). The extent of foxi1 expression (bracket) closely correlates with the extent of fgf3 and fgf8 expression. (C) Transverse sections (level of cross sections indicated by a dashed line in (B) revealed fgf3 and fgf8 expression in the mesoderm underlying ectoderm (arrows). Scale bars: 50 μm. Abbreviations are as in Fig. 1; r4, rhombomere 4. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Fgf3 and Fgf8 are necessary for EB placode induction. (A) fgf3+/-;fgf8+/- embryos were crossed to generate various genotypic combinations, including fgf3;fgf8 mutants. Resulting embryos were processed for in-situ hybridization with eya1, foxi1, pax2a, ngn1 and phox2b riboprobes, photographed and genotyped (genotypes are shown in the bottom left of each panels). All panels show lateral views, except eya1-expression panels, which show dorsal views. PPE is not affected in fgf3 or fgf8 mutants or fgf3;fgf8 double mutant. Consistent with our previous observations (Nechiporuk et al., 2005), fgf3 mutants lacked ngn1 and phox2b expression in glossopharyngeal and small vagal ganglia, whereas foxi1 and pax2a expression was normal. All markers were expressed normally in fgf8-/- embryos. However, foxi1 expression (brackets) was strongly reduced and pax2a, ngn1, and phox2b expression was either absent or strongly reduced in fgf3;fgf8 double mutants. Vagal neural crest is not affected in fgf3;fgf8 double mutants (arrowheads). fgf3+/-;fgf8-/-embryos displayed intermediate phenotypes, where expression of all markers were reduced but not absent. (B,C) Transverse sections of fgf3/8 double mutants and wild-type siblings were obtained from the whole-mounts processed for foxi1 (B) and pax2a (C) in situ (dashed lines in A indicate level of cross section). In contrast to wild type, the epithelium appears disorganized and foxi1 expression is not restricted to a single cell layer in fgf3;fgf8 mutants. pax2a expression is absent from the ectoderm in fgf3;fgf8 mutants. Scale bars: 50 μm. Abbreviations are as in Fig. 1; a, acoustic ganglion; al, anterior lateral line ganglion; op, otic placode. PHENOTYPE:
|
|
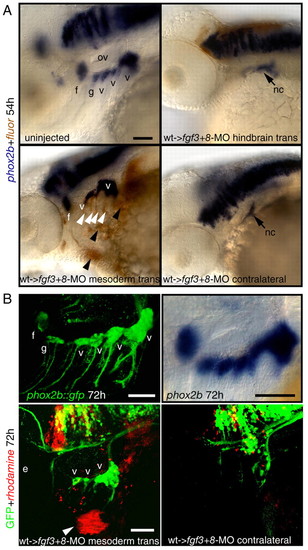
Restoration of cephalic mesoderm is sufficient to rescue EB ganglia. Wild-type donor embryos were injected with a lineage tracer fluorescein (A, brown), or rhodamine (B, red). At 30-40% epiboly, 25-30 donor cells were transplanted into the margin of fgf3+8-MO hosts. Mosaic embryos were collected at 54 (A) or 72 (B) hpf and analyzed for phox2b (blue, A) or GFP expression (green, B), respectively. (A) Lateral views of an uninjected control embryo (top, left) or fgf3+8 hosts that received hindbrain or mesodermal transplant. Note that mesodermal wild-type donor cells (brown stain, black arrowheads) efficiently rescued facial and vagal ganglia when compared with the contralateral control. Some donor cells also contributed to pharyngeal endoderm (white arrowheads). (B) phox2b::egfp transgene (top, left) recapitulates phox2b expression in the EB ganglia when compared with the endogenous message (top, right). Lateral view of the 72-hour old mosaic phox2b::egfp embryo that showed rescue of the vagal ganglia (compare with the contralateral side on the right). Donor cells (red, arrowhead) contributed to the facial muscles, indicating mesodermal origin. Note that in this example donor cells did not contribute to the endodermal pouches. Scale bars: 50 μm. Abbreviations are as in Fig. 1; ov, otic vesicle. |
|
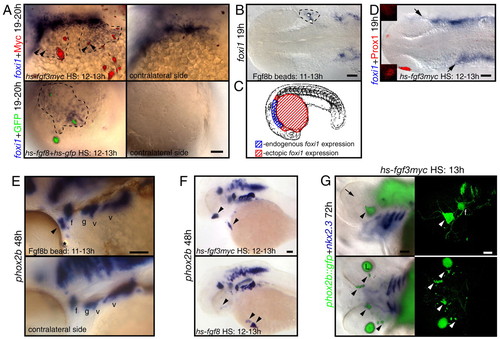
Fgf3 or Fgf8 is sufficient to induce foxi1-positive EB precursors and phox2b-positive EB neurons in wild-type embryos. Zebrafish embryos were injected with hs-fgf3myc or coinjected with hs-fgf8 and hs-gfp plasmids at one-cell stage, heat shocked at 10-13 hpf and then assayed for foxi1 or phox2b expression (blue). Alternatively, embryos were implanted with Fgf8b-coated beads at 11 hpf and assayed for foxi1 and phox2b expression at 19 and 48 hpf, respectively. (A,E,F) Lateral views; (B,D) dorsal views. (A) Ectopic foxi1-positive cells (outlined by dotted line) are immediately adjacent to the Myc-(red) or GFP-positive (green) cells expressing Fgf. Note punctate staining surrounding Myc-positive cells, presumably indicating secreted myc-tagged Fgf3 protein. (B) Ectopic foxi1 expression (dotted line) was induced in the vicinity of Fgf8b beads. (C) Summary of the ectopic Fgf expression experiments. Ectopic foxi1 foci (shown in red) were restricted to the ventral side of the yolk surface, just ventral and anterior to the endogenous foxi1-expression domain. (D) Activation of hs-fgf3myc leads to the anterior expansion of foxi1 domain (arrows) and loss of Prox1 expression in the lens (red). Insets show lateral views of the presumptive lens domain on each side of the embryo. (E) Fgf8b bead (star) induced formation of the ectopic phox2b-positive EB neurons (arrowhead). (F,G) Activation of Fgf3myc or Fgf8 in wild-type (F) or phox2b::egfp transgenic embryos (G) induced ectopic phox2b-positive EB neurons (arrowheads) away from the endogenous phox2b-expression sites. (G) Left panels show overlay of immunofluorescence and brightfield photographs, and right panels show confocal stacks generated from the same embryos. Note ectopic phox2b-positive cells on the ventral surface of the head as well as in the eye (arrowheads). Formation of the ectopic phox2b-positive neurons (green) did not require endoderm pouch tissue, visualized by nkx2.3 (purple). Note complete absence of lens tissue (arrow, |
|
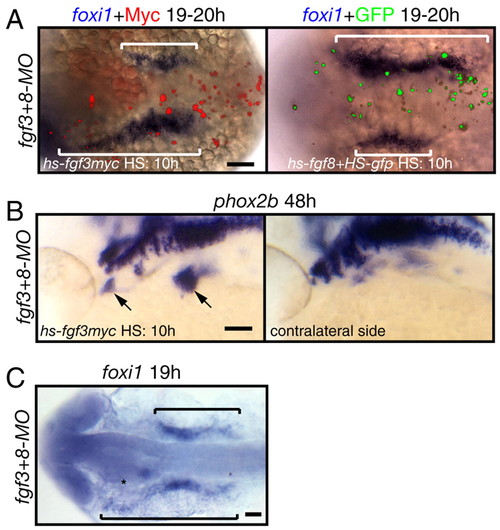
Fgf3 or Fgf8 is sufficient to induce foxi1-positive EB precursors and phox2b-positive EB neurons in fgf3+8 morphants. (A,C) Dorsal views; (B) lateral views. (A,B) Zebrafish embryos were injected with hs-fgf3myc or co-injected with hs-fgf8 and hs-gfp plasmids at one-cell stage, heat shocked at 10-13 hpf and then assayed for foxi1or phox2b expression (blue) and Myc or GFP expression (red and green, respectively). Fgf3myc or Fgf8 efficiently rescued foxi1 (brackets in A) or phox2b (arrows in B) expression in fgf3+8 morphants. Note the difference between foxi1 expression domain adjacent to Myc or GPF-expressing cells and contralateral side. (C) fgf3+8 morphant |
|
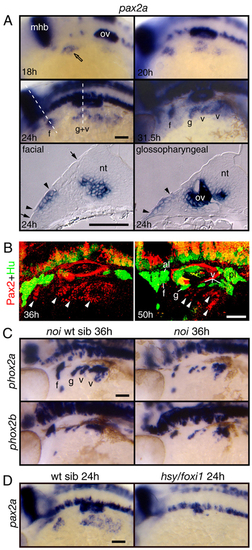
Pax2a is expressed in EB placodes and is required for EB placode development. (A) Expression analyses of pax2a at 18, 20, 24 and 31.5 hpf. Whole-mount panels are lateral views of the embryos; transverse sections are at the level of the facial placode and glossopharyngeal placodes. pax2a expression begins in the presumptive facial placode at 18 hpf (open arrow). At 20-24 hpf, its expression extended caudally to include presumptive glossopharyngeal and vagal placodes. Between 28 and 31.5 hpf, pax2a expression was maintained in the individual placodes and internalized neuroblasts. Transverse sections at 24 hpf revealed the presence of pax2a mRNA (arrowheads) in the thickened columnar epithelium (the extent of the thickened epithelium is marked by arrows), a hallmark of cranial placodes. Interestingly, the facial domain of pax2a expression was restricted to the ventral part of the epithelium, indicating that the EB placodes are restricted to the ventral part of the competent ectoderm. (B) Zebrafish embryos were collected at 36 and 50 hpf, and processed for immunolabeling with Pax2 (red) and Hu (green) antibody. Pax2a protein (arrowheads) was downregulated in the differentiated Hu-positive EB neurons. (C) Pax2a activity is required during EB ganglia development. The zebrafish pax2a mutant no isthmus (noi) displayed a reduction in EB neurons. (D) Foxi1 activity is required for pax2a expression. Notice that pax2a message is absent in the hsy/foxi1-mutant embryos. a, acoustic ganglion; al, anterior lateral line ganglion; f, facial placode or ganglion; g, glossopharyngeal placode or ganglion; mhb, midhindbrain boundary; nt, neural tube; ov, otic vesicle; pl, posterior lateral line ganglion; t, trigeminal ganglion; v, vagal placode or ganglion. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Fgf signaling is active in EB placode at the time of induction. Expression analyses of fgfr1, fgfr2, erm and pea3 at 13-14 hpf. Whole-mount panels (left) are dorsal views of the embryos; transverse sections (right) are at the level of r2-r4. Notice expression of these markers just lateral to the neural tube (arrowheads). Transverse sections revealed ectodermal expression of fgfr1, erm, and pea3 (arrows). Scale bars: 50 μm. EXPRESSION / LABELING:
|
|
Mesodermal fgf3 and fgf8 expression is absent in MZoep mutants. MZoep mutants and age-matched controls were analyzed for fgf3 and fgf8 expression at 15 hpf. All panels show dorsal views. Mesodermal expression (arrowheads) of fgf3 and fgf8 was absent (bottom panels), whereas CNS expression was largely unaffected in MZoep mutants. mhb, midhindbrain boundary; r4, rhombomere4. Scale bar: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
fgf3+8 morphants phenocopy fgf3/8 double mutant phenotype. Zebrafish embryos injected with a fgf3 and fgf8 morpholino alone or together and uninjected controls were collected at 36 hpf and processed for in situ hybridization with phox2b riboprobe. All panels show lateral views. Notice that injection of the suboptimal amounts of fgf3-MO alone causes only a slight reduction in the EB ganglia. However, injection of the same amount of fgf3-MO together with fgf8-MO causes complete absence of the EB ganglia. Also notice that the locus coeruleus is completely eliminated in fgf3+8 morphants, allowing the efficiency of injections to be monitored. a, acoustic ganglion; al, anterior lateral line ganglion; f, facial placode or ganglion; g, glossopharyngeal placode or ganglion; mhb, midhindbrain boundary; nt, neural tube; ov, otic vesicle; pl, posterior lateral line ganglion; t, trigeminal ganglion; v, vagal placode or ganglion; lc, locus coeruleus; nc, vagal neural crest. Scale bars, 50 μm. |