- Title
-
Cyclooxygenase-1 signaling is required for vascular tube formation during development
- Authors
- Cha, Y.I., Kim, S.H., Solnica-Krezel, L., and Dubois, R.N.
- Source
- Full text @ Dev. Biol.
|
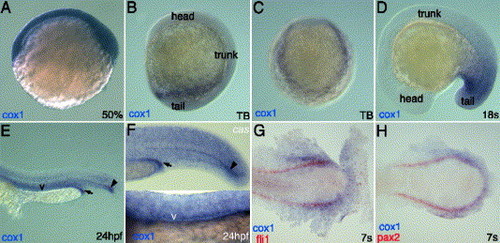
cox1 transcripts localize to posterior mesoderm organs. Whole mount in situ hybridization of cox1 (A–H) reveals that its transcripts are ubiquitously present at shield stage (A) and localize posterior half of the embryos at tailbud stage (B–C) from side view (B) and from posterior view (C). cox1 co-localizes with pax2 (H) and fli1 (G) at 7 somite stage, indicating its expression in posterior intermediate mesoderm and posterior lateral mesoderm. During late somitogenesis stage, cox1 transcripts strongly stain posterior mesoderm (D). At 24 hpf, cox1 can be seen in various mesodermal structures (E). Expression of cox1 is not altered in casanova mutant, deficient in endoderm derivatives (F). Arrow indicates the distal end of pronephric duct, letter v indicates the vasculature and arrowhead points to the distal hypochord. Scale bar: 100 μm. |
|
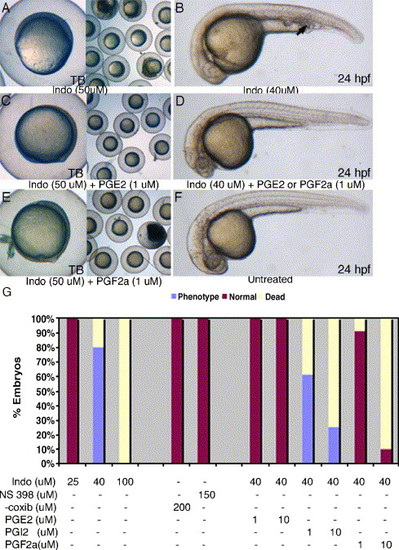
PGE2 and PGF2α can rescue both gastrulation arrest and posterior mesoderm defects induced by indomethacin. Indomethacin treatment results in gastrulation arrest at 50 μM (A) and posterior mesoderm defects at 40 μM (B, arrow). Supplementing indomethacin with PGE2 or PGF2α can suppress both the gastrulation arrest (C, E) and posterior mesoderm defects (D). COX-2 inhibitors, NS-398, or celecoxib do not cause any noticeable phenotypic defects up to 150 μM (G). Two independent experiments were performed (n > 80) in indomethacin, celecoxib, and NS-398 treatment groups. In rescue experiments, we performed three independent experiments (n > 80) in each of the treatment groups. |
|
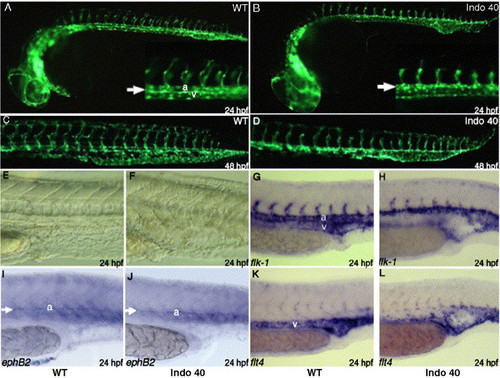
COX-1 inhibition causes defect in vascular tube formation. Fli1:EGFP transgenic embryos were treated with 40 μM indomethacin at tailbud stage. Compared to untreated embryos (A, C), indomethacin treatment results in distended posterior vasculature at day 1 (B, arrow). Posterior vasculature cannot differentiate into dorsal aorta and posterior cardinal vein (B, inset arrow). At day 2, clear distinction between dorsal aorta and caudal vein can be seen in untreated embryo (C), but a single distended posterior vessel remains in treated embryo (D). In addition, intersomitic vessels are either shortened or absent at day 1 (B, inset) and results in shortened intersomitic vasculature at day 2 (D). Indomethacin treatment results in distention of posterior vasculature at day 1 (F), compared to untreated embryos (E). Staining with flk1, an endothelial marker, reveal the significant reduction or absence of venous staining at day 1 in treated embryos (H), compared to the wild-type (G). While ephB2, arterial marker, staining shows similar staining between wt (I) and treated embryos (J), flt4, vein marker, staining shows absence of veinous staining, especially in the arterial–venous boundary (L). EXPRESSION / LABELING:
|
|
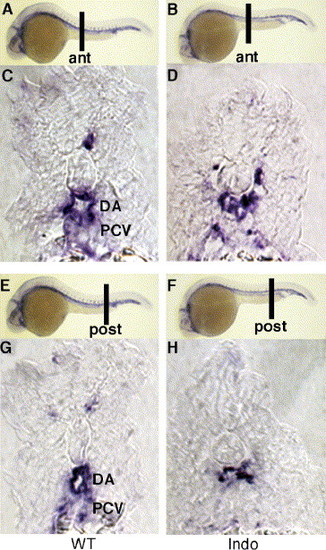
COX-1-inhibited embryos are unable to form distinct dorsal aorta and posterior cardinal vein. Cross-section of anterior (A, C) and posterior trunk (E, G) in untreated embryos displays distinct dorsal aorta and posterior cardinal vein. Indomethacin-treated embryos do not form two vascular tubes but display one distended vessel both in anterior (B, D) and posterior sections (F, H). ant, anterior; post, posterior. All embryos were sectioned after staining with flk1. EXPRESSION / LABELING:
|
|
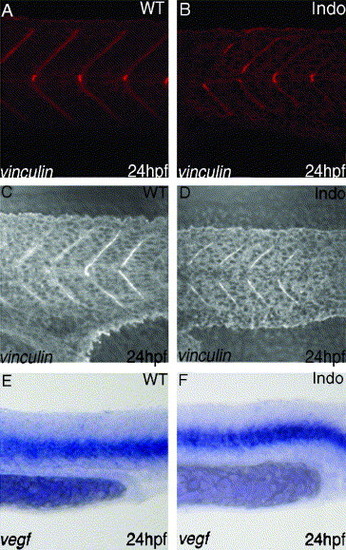
Inhibition of COX-1 does not cause defect in somite formation or VEGF production. Vinculin staining, marker for intersomitic boundary, reveals normal somite formation in the trunk both in control group (A) and treated group (B). In the tail region, vinculin staining is overlayed on DIC image of the same area. It also reveals that somite formation of the tail is unchanged in control group (C) vs. treated group (D). Somites in the treated embryos produce normal amounts of VEGF (F), compared to the untreated group (E). EXPRESSION / LABELING:
|
|
COX-1 signaling is required at 25 somite stage for posterior vessel formation. We monitored the presence of posterior vessel defect at 38 hpf after starting indomethacin treatment at various stages. Starting treatment at 20 s, 25 s, 25 hr or 30 hpf to 38 hpf all resulted in posterior vessel defect. Starting treatment at 5 s to any stage before 25 somites resulted in absence of posterior vessel defect, whereas ending the treatment at 28 hpf and 32 hpf resulted in posterior vessel defect in 35% and 80% of the embryos respectively. Red line indicates timing of indomethacin treatment. |
|
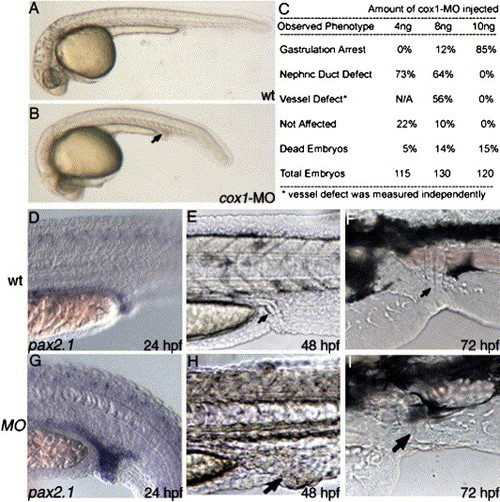
cox1-MO causes posterior mesoderm defect similar to indomethacin-induced phenotype. Compared to uninjected wild-type (A), injection of 8 ng cox1-MO (B) specifically disrupted posterior mesoderm organs at 1 day, including enlarged nephric duct and distended posterior vasculature (B, arrow). Staining with pax2.1 shows increased pronephric duct size in the posterior region (D). As the embryos develop, the increased staining correlates to increase in posterior nephric duct size at day 2 (H, arrow) and day 3 (I, arrow), compared to wild-type (E, F). EXPRESSION / LABELING:
|
|
COX-1 knockdown results in gastrulation defect at high dose. Injection of 10 ng cox1-MO results in gastrulation arrest at ~50% epiboly stage (A). Gastrulation arrest defect by cox1-MO can be rescued by co-injecting synthetic cox1 RNA, mutated in MO-binding site (B). Black bar indicates the percentage of embryos that survived to tailbud (C). In the rescue experiments (lane 3, 4), we tested at least 80 embryos from two independent experiments, while in cox1 RNA injection groups (lane 5, 6), at least 50 embryos from two independent experiments were analyzed. |
|
Vessel defect is specific for posterior vasculature. Anterior vasculature appears to progress normally since flk1 staining in indomethacin-treated embryos (B) does not differ from untreated embryos (A). However, in the posterior vasculature, caudal vein is absent in the indomethacin treatment embryos (D arrow), while untreated embryos (C) show continuous flk1 throughout posterior vasculature. |
Reprinted from Developmental Biology, 282(1), Cha, Y.I., Kim, S.H., Solnica-Krezel, L., and Dubois, R.N., Cyclooxygenase-1 signaling is required for vascular tube formation during development, 274-283, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.