- Title
-
Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish
- Authors
- Wehner, D., Tsarouchas, T.M., Michael, A., Haase, C., Weidinger, G., Reimer, M.M., Becker, T., Becker, C.G.
- Source
- Full text @ Nat. Commun.
|
Regenerating axons navigate a non-neural lesion environment that is rich in ECM independently of astroglia-like processes. a Time-lapse video-microscopy reveals that axonal growth cones (arrow, Xla.Tubb:DsRED) extend into the lesion site independently of astroglia-like processes (her4.3:EGFP, empty arrow points out lack of glial labelling). Arrowhead indicates glial process extending into the lesion site. Single frames are shown. Abbreviations: fin, dorsal fin fold; sc, spinal cord; nc, notochord. b Quantification of fascicle composition at 1 dpl using different combinations of immunohistochemical and transgenic markers for astroglia-like processes and axons indicates that the majority of fascicles are neuronal with no detectable glial component (46–62% of all fascicles analyzed). In the example scans on the left, yellow arrows indicate mixed fascicles, containing neurites and glial processes; green arrows indicate pure glial fascicles and red arrows indicate pure axonal fascicles. c Time course of re-growth of axons (anti-acetylated Tubulin+) and astroglia-like processes (anti-GFAP+) after spinal cord transection. Quantification of labelling continuity between rostral and caudal spinal cord stumps at 1 dpl and 2 dpl suggests faster bridging of the lesion site by neuronal rather than glial processes. Arrow points to axonal bridge with very few glial processes in the lesion site at 1 dpl at higher magnification (Fischer’s exact test: **P < 0.01; n.s. indicates not significant). d Treatment of gfap:Gal4ff;UAS:NTR-mCherry transgenic fish with the pro-drug Metronidozole (MTZ) almost completely ablates transgene-expressing glial cells by 3 dpf. e Treatment of gfap:Gal4ff;UAS:NTR-mCherry transgenic fish with MTZ dramatically reduces anti-GFAP+astroglia-like processes (t-test: ***P < 0.001) but does not reduce the proportion of larvae with axonal bridges at 2 dpl (Fischer’s exact test: n.s indicates not significant). f fn1a and col1a2 expression is upregulated after lesion, shown by in situ hybridization. g Regenerating axons (anti-acetylated Tubulin+; arrows) are closely associated with Collagen I and Fibronectin immunoreactivity in a lesion site (confocal depth was limited to spinal cord). Also see Supplementary Movies 3 and 4. a–g Views are dorsal (a; rostral is left) or lateral (b-g; dorsal is up, rostral is left). BF: brightfield. Scale bars: 100 µm a,c–f and 50 µm b,g. Error bars indicate s.e.m EXPRESSION / LABELING:
PHENOTYPE:
|
|
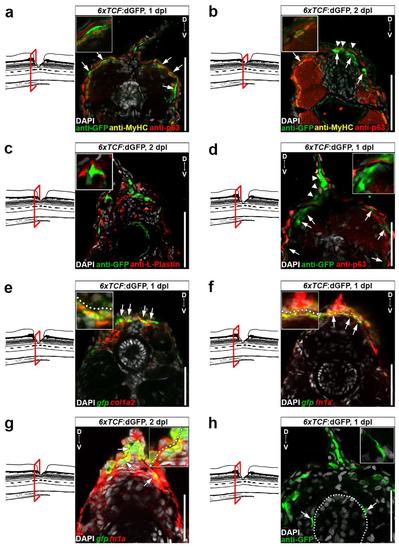
Wnt/β-catenin signaling is mainly active in fibroblast-like cells after spinal cord lesion. a Cartoon showing relevant transcriptional read-outs of Wnt/β-catenin pathway activity, feedback regulatory mechanisms and strategies to interfere with signaling at different levels of the pathway. Abbreviation: β-cat., β-catenin. b Detection of gfp mRNA in the 6xTCF:dGFP transgenic reporter line shows transient activity of the Wnt/β-catenin pathway in the lesion site during regeneration. c gfp expression is largely confined to the lesion edge in 6xTCF:dGFP transgenic larvae (arrow). A few additional cells are labelled within the presumptive spinal cord region (empty arrows). Abbreviations: nc, notochord; sc, spinal cord; fin, dorsal fin fold. d The 6xTCF:dGFP Wnt reporter is mostly active in subepidermal p63− cells (arrows; ≥ 69% of all 6xTCF:dGFP+ cells at 1 dpl and 2 dpl), but also in p63+ basal keratinocytes (arrowheads) in the lesion site. # indicates non-specific signal. e Consistent with a fibroblast identity, subepidermal cells (below dashed line) co-express col1a2 (red) and gfp mRNA (green) in a lesioned 6xTCF:dGFP transgenic animal (arrow). Dashed line indicates junction between basal epithelium and adjacent connective tissue. f Fibroblast-like (6xTCF:dGFP+/p63−) cells are found in close proximity to regenerating axons (anti-acetylated Tubulin+) in the spinal lesion site at 1 dpl (arrows). A single optical section is shown. a–f Views are lateral (b,c,f; dorsal is up, rostral is left) or transversal (d,e; dorsal is up) as indicated. BF: brightfield. Scale bars: whole mounts, 100 µm b,c and 25 µm f; sections: 100 µm. Error bars indicate s.e.m |
|
Wnt/β-catenin signaling in non-neural cells is required for axon regeneration and functional recovery. a Schematic timeline of the experimental design using the TetON system. b–d axin1 overexpression selectively in cells in the lesion site (b; lef1 +) is sufficient to interfere with axon regeneration (c; Fischer’s exact test: ***P < 0.001) and functional recovery (d; two-way ANOVA: F1,156 = 14.82, P < 0.0005; t-test: ***P < 0.001). e–g axin1 overexpression throughout the fish e interferes with axon regeneration (f; Fischer’s exact test: *P < 0.05) and functional recovery (g; two-way ANOVA: F 1,129 = 4.716, P < 0.05; t-test: ***P < 0.001). h–j Neuronal axin1 overexpression, indicated by YFP labelling of parenchymal cell bodies and neuropil in the spinal cord h, does not interfere with axon regeneration i or functional recovery (j; two-way ANOVA shows no interaction between lesion and treatment). k–m axin1 overexpression in ependymoradial glial cells, indicated by YFP labeling in cell bodies lining the central canal, their radial processes and pial end feet k does not interfere with axon regeneration l or functional recovery (m; two-way ANOVA shows no interaction between lesion and treatment). b–e Views are transversal (b,e,h,k; dorsal is up) or lateral (c,f,i,l; dorsal is up, rostral is left). BF: brightfield. n.s.: not significant. Scale bars: whole mounts, 200 and 100 µm; sections: 100 µm. Error bars indicate s.e.m |
|
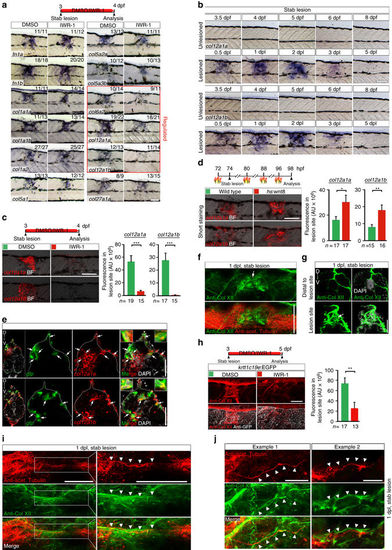
Wnt/β-catenin signaling controls col12a1a/b transcription and Col XII deposition in a spinal lesion site. a Wnt/β-catenin pathway inhibition (IWR-1) results in lower expression of select collagen genes (red box) in the lesion site at 1 dpl, determined by in situ hybridization. b col12a1a and col12a1b expression is transiently upregulated in the lesion site during regeneration. c Inhibition of Wnt/β-catenin signaling (IWR-1) interferes with col12a1a/b expression in the lesion site (t-test: ***P < 0.001). d wnt8 overexpression upregulates col12a1a/b expression in the lesion site in hs:wnt8 transgenic fish (t-test: *P < 0.05, **P < 0.01). Signal was underdeveloped to optimize detection of differences in signal strength. e A subpopulation of col12a1a or col12a1b-expressing cells (red) in the lesion site co-labels with transcripts of the 6xTCF:dGFP Wnt reporter (green, arrows). f,g Anti-Col XII immunoreactivity is increased in the lesion site (arrows) compared to adjacent unlesioned trunk tissue at 1 dpl. Shown is a confocal image of a whole mount (f, confocal depth was limited to spinal cord) and a transversal section though the lesion site g,h Inhibition of Wnt/β-catenin signaling (IWR-1) interferes with Col XII deposition in the lesion site (t-test: **P < 0.01). Note continuity of tissue in IWR-1-treated animals as shown by re-epithelialization of the lesion site by basal keratinocytes in krtt1c19e:EGFP transgenic fish. i,j Double immunolabelling of axons (anti-acetylated Tubulin+) and Col XII shows Col XII matrix deposition in the lesion site through which regenerating axons grow i. Shown is a maximum intensity projection in i with inset higher magnification; the confocal depth was limited to spinal cord. Arrowheads point to regenerating axonal fascicle in close apposition to Col XII immunoreactivity (also see Supplementary Movie 5). Note, that the trajectory of axonal fascicles appear to follow longitudinal fibres of Col XII immunoreactive ECM material in the lesion site (j, arrowheads). a–j Views are lateral (a–d,f,h–j; dorsal is up, rostral is left) or transversal (e,g; dorsal is up). BF: brightfield. Scale bars: 100 µm a–d,f,h, 50 µm e,g,i, 25 µm j. Error bars indicate s.e.m EXPRESSION / LABELING:
PHENOTYPE:
|
|
Knockdown of col12a1a/b inhibits functional axon regeneration. a Cartoon showing strategy used for systemic delivery of Vivo-Morpholino (MO), which acts as cell-permeable translation blocking anti-sense molecules. MOs against col12a1a and col12a1b were co-injected into the cardiac ventricle at 3 dpf, 4 h before lesion. b col12a1a/b MO injection strongly reduces Col XII immunoreactivity in the lesion site compared to control MO injected animals. (t-test: ***P < 0.001). c col12a1a/b MO injection inhibits axon regeneration after lesion (Fischer’s exact test: ***P < 0.001). d col12a1a/b MO injection interferes with functional recovery after lesion (two-way ANOVA: F1,116 = 5.534, p < 0.05; t-test: ***P < 0.001). a–d Lateral views are shown (dorsal is up, rostral is left). BF: brightfield. n.s.: not significant. Scale bars: 200 µm c and 100 µm (b,c). Error bars indicate s.e.m |
|
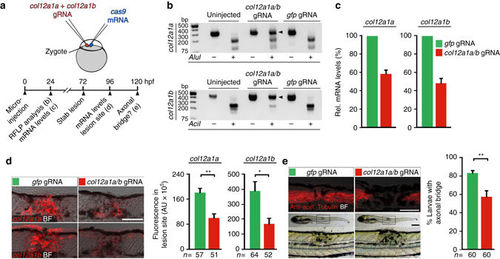
CRISPR/Cas9-mediated disruption of col12a1a/b leads to depletion of mRNA and impaired axonal regeneration. a Cartoon showing strategy used for CRISPR/Cas9-mediated col12a1a/b gene disruption. cas9 mRNA and small guide-RNA (gRNA) targeting col12a1a and col12a1b were co-injected into the zygote. Crispants were analysed for col12a1a/b mRNA levels and axonal regeneration as indicated in the timeline. b Restriction fragment length polymorphisms (RFLP) analysis reveals efficient somatic mutation in the gRNA target site (indicated by resistance to restriction endonuclease digest; arrowheads) in both col12a1 paralogs after microinjection. c col12a1a/b Crispants exhibit reduced developmental col12a1a and col12a1b mRNA expression, determined by quantitative RT-PCR. Representative plot from three independent experiments for each mRNA are shown. mRNA levels in gfp gRNA-injected control animals were set to 100%. d col12a1a/b Crispants exhibit reduced col12a1a and col12a1b mRNA levels after lesion, determined by quantification of fluorescent in situ hybridization signal in the lesion site (t-test: **P < 0.01, *P < 0.05). e col12a1a/b Crispants show reduced axonal bridging events after lesion (t-test: **P < 0.01). a–e Views are lateral (dorsal is up, rostral is left). BF: brightfield. Scale bars: 200 µm e and 100 µm d,e. Error bars indicate s.e.m |
|
Overexpression of col12a1a promotes axon regeneration in Wnt/β-catenin pathway-inhibited and wild type zebrafish larvae. a cerulean mRNA is robustly detectable 24 h after the last heat shock in hs:col12a1a-p2a-Cerulean transgenic larvae. b col12a1a overexpression increases anti-Col XII immunoreactivity in the lesion site of Wnt/β-pathway-inhibited (IWR-1 treatment) hs:col12a1a transgenic larvae (arrow; t-test: ***P < 0.001). c col12a1a overexpression rescues axon regeneration in Wnt/β-catenin pathway-inhibited (IWR-1 treatment) hs:col12a1a transgenic larvae (Fischer’s exact test: ***P < 0.001). d col12a1a overexpression improves functional recovery in Wnt/β-catenin pathway-inhibited (IWR-1 treatment) hs:col12a1a transgenic larvae (t-test: ***P < 0.001). e col12a1a overexpression is sufficient to augment axon regeneration (Fischer’s exact test: *** P< 0.001). a–e Views are lateral (dorsal is up, rostral is left). BF: brightfield. Scale bars: 200 µm c and 100 µm a–c, e. Error bars indicate s.e.m |
|
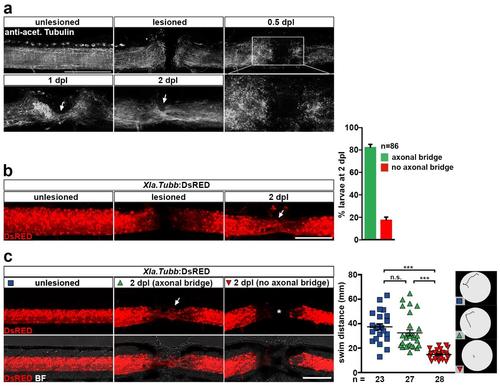
Functional spinal cord regeneration correlates with axon regrowth in zebrafish larvae. (a) Time-course of axon regrowth (anti-acetylated Tubulin+) after spinal cord transection. Note that at 0.5 dpl (12 hpl) little axon regrowth is evident and mainly axonal debris is found in the lesion site (inset). At 1 dpl axons begin to bridge the lesion, which is has substantially progressed at 2 dpl (arrows). (b) Repetitive imaging of Xla.Tubb:DsRED transgenic larvae shows axonal bridge formation across the lesion site (arrow) in >80% of all animals analysed. Shown is the same animal before lesion, immediately after lesion and at 2 dpl. (c) Functional recovery, as measured by the swim distance after touch, correlates with axon regrowth across the lesion site (axonal bridge formation) in larval zebrafish. Animals with an axonal bridge at 2 dpl (arrow) cover similar swim distance as unlesioned age-matched control animals. In animals in which no axonal reconnection is established by 2 dpl (asterisk), recovery of swim distance is impaired (one-way ANOVA with Dunn’s multiple comparison test: ***P<0.001, n.s. indicates not significant). Example swim tracks are shown. (a-c) Views are lateral (dorsal is up, rostral is left). BF: brightfield. Scale bars: 200 μm (c) and 100 μm (a-b). Error bars indicate s.e.m. |
|
Wnt/β-catenin pathway is mainly active in the spinal lesion site. (a) Expression of the transgenic Wnt/β-catenin pathway reporter 7xTCF:mCherry and Top:dGFP is upregulated after lesion. (b) Expression of direct Wnt target genes axin2 and lef1 is upregulated after lesion. (c) 6xTCF:dGFP Wnt reporter activity in the spinal lesion site is detectable 12 hours (0.5 dpl) after a stab lesion or dorsal incision lesion (arrows). Note that larvae were treated with PTU to suppress pigmentation. (d) Detection of gfp mRNA in the 6xTCF:dGFP transgenic reporter line shows transient activity of the Wnt/β-catenin pathway in the lesion site during regeneration, analysed in stab-lesioned animals. (e) Representative images of a dorsal incision lesion or less invasive stab lesion taken immediately after lesion. Note that stab lesions do not compromise the dorsal edge of the animal, resulting in reduced injury size as compared to dorsal incision lesions. Arrowhead points to the lesion site. (f) 6xTCF:dGFP Wnt reporter activity in the lesion site is strongly reduced upon IWR-1 treatment for 12 hours, indicating specificity of the transgenic Wnt reporter. (g) 6xTCF:dGFP Wnt reporter activity in the lesion site is strongly reduced upon heat shock-induced axin1 overexpression for 6 hours in 6xTCF:dGFP;hs:Axin1 double transgenic animals, indicating specificity of the transgenic Wnt reporter. (h) GFP protein is largely confined to the lesion site in 6xTCF:dGFP transgenic animals at 1 dpl and 2 dpl. A few additional cells are labelled within the presumptive spinal cord region in the periphery of the lesion site (empty arrows). Maximum intensity projections and a single optical section through the center of a whole mount larvae are shown. (i) Anti-GFP immunohistochemistry on sections of 6xTCF:dGFP transgenic animals confirms labelling in whole mount preparations. 6xTCF:dGFP Wnt reporter activity at 2 dpl is largely confined to the lesion site. In the peripheral lesion area limited Wnt reporter activity is also detected in the spinal cord (empty arrows). (j) Detection of gfp mRNA by in situ hybridization in 5 day-old her4.3:EGFP transgenic zebrafish, labelling ependymoradial glia cells, reveals efficient probe penetration in whole mount preparations. (a-j) Views are lateral (a-h, j; dorsal is up, rostral is left) or transversal (i; dorsal is up). BF: brightfield. Scale bars: whole mounts, 200 μm (j,e) and 100 μm (a-d, f-h); sections, 100 μm. |
|
Wnt/β-catenin pathway is upregulated in basal keratinocytes and fibroblast-like cells but not muscle or immune cells after a lesion. (a-b) Subepidermal 6xTCF:dGFP+/p63− cells (arrows) do not co-label with MyHC antibodies, a marker for slow twitch muscle cells. Arrowheads indicate 6xTCF:dGFP+/p63+ basal keratinocytes. Note that close to the disorganized lesion center some subepidermal 6xTCF:dGFP+/p63+ cells are found sandwiched between p63+ basal keratinocytes and MyHC+ slow twitch muscle fibers (insets), which represents a typical dermal fibroblast location in zebrafish larvae. (c) 6xTCF:dGFP+ cells do not co-label with L-Plastin, a marker for innate immune cells. (d) The 6xTCF:dGFP Wnt reporter is active in p63+ basal keratinocytes (arrowheads) and in subepidermal p63+ cells (arrows) in the lesion site. (e) Subepidermal cells (below dashed line) co-express col1a2 (red) and gfp mRNA (green) in lesioned 6xTCF:dGFP transgenic animals (arrow). Dashed line indicates junction between basal epithelium and subadjacent connective tissue. (f-g) Subepidermal cells (below dashed line) co-express fn1a (red) and gfp mRNA (green) in lesioned 6xTCF:dGFP transgenic animals (arrow). Dashed line indicates junction between basal epithelium and subadjacent connective tissue. (h) Close to the disorganized lesion center, some 6xTCF:dGFP+ cells are found to cover the spinal cord (arrows), which represents a typical meningeal fibroblast location. Dashed line indicates spinal cord. (a-h) Views are transversal (dorsal is up). Scale bars: 100 μm (a-c) and 50 μm (d-h). |
|
Utilizing the TetON system for cell type-specific Wnt/β-catenin pathway manipulation. (a) The TetON system allows for inducible transgene expression. YFP fluorescence (TetResponder transgene) is robustly induced in DOX-treated but not EtOH-treated ubi:TetA AmCyan;TetRE:Axin1-YFP double transgenic animals. (b) Consistent with Wnt/β-catenin pathway activation, AmCyan fluorescence is undetectable in unlesioned lef1:TetA AmCyan transgenic animals and upregulated after a lesion (arrow). (c) gfp mRNA (green) and amcyan mRNA (red) are co-expressed in lesioned 6xTCF:dGFP;lef1:TetA AmCyan double transgenic animals, indicating that lef1 regulatory elements drive gene expression in Wnt-responding cells in the lesion site. (d) DOX treatment of lef1:TetA AmCyan;TetRE:Axin1-YFP double transgenic animals (but not single transgenic control animals) interferes with axin2 expression in non-neural lesion site cells. (e) Ubiquitous axin1 overexpression through DOX treatment of ubi:TetA AmCyan; TetRE:Axin1-YFP double transgenic animals, interferes with axin2 expression in non-neural lesion site cells. (f) axin1 overexpression specifically in neurons through DOX treatment of Xla.Tubb:TetA AmCyan;TetRE:Axin1-YFP double transgenic animals does not reduce axin2 expression in non-neuronal lesion site cells. (g) DOX treatment of Xla.Tubb:TetA AmCyan;TetRE:Axin1-YFP double transgenic animals (but not single transgenic control animals) reduced axin2 expression in a constitutively Wnt-responsive domain in the brain (arrows), indicating that the Xla.Tubb:TetA AmCyan TetActivator line drives functionally relevant axin1 levels in neurons. (a-g) Views are lateral (dorsal is up, rostral is left). BF: brightfield. Scale bars: whole mounts, 200 μm (g), 100 μm (a-b, d-f) and 25 μm (c). |
|
Different lesion and manipulation paradigms support that Wnt/β-catenin signaling is required for axon regeneration and functional recovery after spinal cord lesion. (a) Pharmacological interference (IWR-1) with Wnt/β-catenin signaling inhibits axon regeneration (Fischer’s exact test: ***P<0.001) and functional recovery (two-way ANOVA: F1,98=13.71, P<0.0005; t-test: ***P<0.001) in lesioned animals. (b) Heat shock-induced ubiquitous overexpression of the Wnt/β-catenin pathway antagonist axin1 inhibits axon regeneration (Fischer’s exact test: ***P<0.001) and functional recovery (two-way ANOVA: F1,161=5.319, P<0.05; t-test: ***P<0.001) in lesioned hs:Axin1 transgenic animals. (c) Heat shock-induced ubiquitous overexpression of the Wnt/β-catenin pathway antagonist dkk1 inhibits axon regeneration (Fischer’s exact test: ***P<0.001) in lesioned hs:dkk1 transgenic animals. (d) Pharmacological interference (IWR-1) with Wnt/β-catenin signaling starting at different time points post-lesion indicates that pathway activity is required between 0.5 dpl (12 hpl) and 1 dpl for axon regeneration (Fischer’s exact test: *P<0.05, ***P<0.001, n.s. indicates not significant). (e) Pharmacological (IWR-1) interference with Wnt/β-catenin signaling inhibits axon regeneration (Fischer’s exact test: ***P<0.001) and functional recovery (two-way ANOVA: F1,80=8.708, P<0.0005) in stab-lesioned animals. (a-e) Views are lateral (dorsal is up, rostral is left. BF: brightfield. Scale bars: whole mounts, 200 μm and 100 μm. Error bars indicate s.e.m. |
|
Wnt/β-catenin signaling is not required for reepithelialization of the lesion site. Pharmacological (IWR-1) interference with Wnt/β-catenin signaling does not prevent re-epithelialization of the lesion site by basal keratinocytes (arrow), visualized in krtt1c19e:EGFP transgenic animals. Maximum intensity projections and single optical sections though the center of a whole mount larvae are shown. Views are lateral (dorsal is up, rostral is left). Scale bar: 200 μm. |
|
Non-cell autonomous Wnt/β-catenin signaling is required for glial bridging. (a) Heat shock-induced ubiquitous overexpression of the Wnt/β-catenin pathway antagonist axin1 inhibits glia growth (visualized in her4.3:mCherry transgenic animals) across the lesion site (Fischer’s exact test: ***P<0.001) in hs:Axin1 transgenic animals. (b) axin1 overexpression specifically in ependymoradial glial cells through DOX treatment of her4.3:TetA AmCyan;TetRE:Axin1-YFP double transgenic fish does not interfere with glial growth across the lesion site (Fischer’s exact test: n.s. indicates not significant). (a-b) Views are lateral (dorsal is up, rostral is left). Scale bar: 100 μm. Error bars indicate s.e.m. |
|
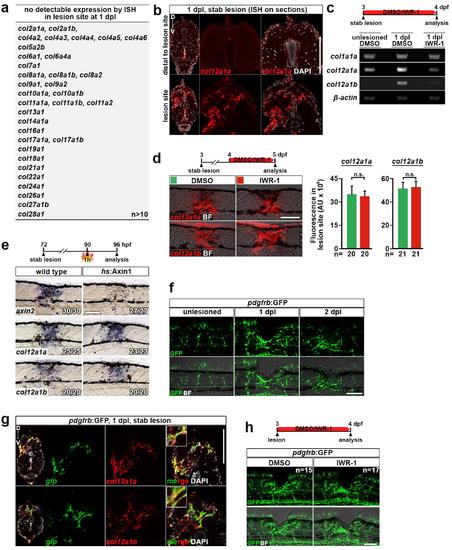
Wnt/β-catenin signaling selectively controls col12a1a/b transcription in a spinal lesion site during the initial phase of axon regeneration. (a) List of genes encoding collagen-chains that were not detectably expressed in the lesion site of untreated or Wnt/β-catenin pathway-inhibited (IWR-1) animals at 1 dpl by in situ hybridization. (b) In situ hybridization (ISH) on section confirms that col12a1a expression is confined to the lesion site. (c) RT-PCR of trunk tissue surrounding the lesion site confirms that lesion-induced upregulation of col12a1a/b expression depends on Wnt/β-catenin signaling. (d) Inhibition of Wnt/β-catenin signaling (IWR-1) between 1-2 dpl does not affect expression of col12a1a/b in the lesion site, as determined by quantification of fluorescence in situ hybridization signal (t-test: n.s. indicates not significant). (e) Expression of axin2, a direct Wnt/β-catenin target, but not of col12a1a or col12a1b is strongly reduced in the lesion site within 6 hours of pathway inhibition by heat shock induced overexpression of axin1 in hs:Axin1 transgenic animals. This suggests indirect transcriptional control of col12a1a/b genes by the Wnt/β-catenin pathway. (f) pdgfrb:GFP-expressing cells accumulate in the lesion site after lesion. (g) The majority of col12a1a/b-expressing cells (red) co-express gfp mRNA (green) in lesioned pdgfrb:GFP transgenic animals (arrow), a marker for pericytes and reactive fibroblasts. (h) Interference with Wnt/β-catenin signaling (IWR-1) does not overtly inhibit appearance of pdgfrb:GFP+ cells. (a-h) Views are lateral (d-f, h; dorsal is up, rostral is left) or transversal (b, g; dorsal is up). BF: brightfield. Scale bars: whole mounts, 100 μm; sections, 100 μm. Error bars indicate s.e.m. |
|
Wnt/β-catenin signaling controls Col XII matrix deposition in a spinal lesion site through which regenerating axons grow. (a) Anti-Col XII immunoreactivity is increased in the lesion site (arrows) at 1 dpl and 2 dpl compared to adjacent unlesioned tissue and to unlesioned animals at the same trunk position. (b) Inhibition of Wnt/β-catenin signaling via heat shock-induced systemic overexpression of the pathway antagonist dkk1 interferes with Col XII deposition in the lesion site, as determined by quantification of lesion site immunoreactivity (t-test: **P<0.01). (c) Double immunolabelling of axons (anti-acetylated Tubulin+) and Col XII shows little to no Col XII deposition in the lesion site at 0.5 dpl (12 hpl) and axon are yet to enter the lesion site (asterisk). At 1 dpl, anti-Col XII immunoreactivity is markedly increased in the lesion site and axon have entered the Col XII-rich lesion site (arrow). Confocal depth was limited to spinal cord. Note, that the 1 dpl dataset is the same as presented in Fig. 4f. (a-c) Views are lateral (dorsal is up, rostral is left). BF: brightfield. Scale bars: whole mounts, 100 μm (a-b) and 50 μm (c). Error bars indicate s.e.m. |
|
Col XII matrix deposition in a spinal lesion site is conserved across developmental stages in zebrafish. (a) In adult zebrafish, col12a1a is strongly expressed in a spinal lesion site (bottom panel). Transcripts are undetectable in the intact spinal cord distal to the lesion site (top panel). Abbreviations: sc, spinal cord; m, muscle; v, vertebra. (b) In adult zebrafish, regenerating axons (anti-acetylated Tubulin+) navigate a Col XII-rich lesion environment (arrows). Note that Col XII immunoreactivity is undetectable in the intact portion of the spinal cord (asterisk). Shown is a maximum intensity projection (82 μm) and single optical sections with higher magnification showing close association between axons and Col XII. Note that the trajectory of many axonal fascicles appear to follow longitudinal fibres of Col XII immunoreactive ECM material in the lesion site (arrowheads). (a-b) Views are transversal (a; dorsal is up) or dorsal (b; rostral is left). Scale bars: 250 μm (a), 200 μm (b). |
|
Systemic administration of col12a1a/b Vivo-Morpholinos does not interfere with Fibronectin or Collagen I deposition in the lesion site. (a) Rhodamine dextran injected into the cardiac ventricle of 3 dpf zebrafish is efficiently distributed in the extracellular space throughout the animal within 30 min after injection. (b) Injection of col12a1a/b Vivo-Morpholinos (MO) does not affect anti-Fibronectin (top panel) or anti-Collagen I (Col I; bottom panel) immunoreactivity in the lesion site (t-test: n.s. indicates not significant). (a-b) Views are transversal (a; dorsal is up) or lateral (b; dorsal is up, rostral is left). BF: brightfield. Scale bars: whole mounts, 100 μm; sections, 100 μm. Error bars indicate s.e.m. |
|
CRISPR/Cas9-mediated disruption of col12a1a/b leads to impaired axonal regeneration. (a) Transient CRISPR/Cas9-mediated gene editing of either col12a1a or col12a1b does not affect axonal regeneration (Fischer’s exact test: n.s. indicates not significant). (b) Lack of axonal regeneration correlates with high gene editing efficiency of both col12a1 paralogues in CRISPR -manipulated larvae. Single larvae were analysed for axonal bridging at 2 dpl followed by restriction fragment length polymorphism (RFLP) analysis and determination of the gene edition efficiency index. Larvae without axonal bridge at 2 dpl show on average higher gene editing efficiency of col12a1a and col12a1b than larvae with axonal bridge (t-test: **P<0.01). Abbreviation: RE, restriction endonuclease. Error bars indicate s.e.m. |
|
Using hs:col12a1a-p2a-Cerulean transgenic fish for inducible col12a1a overexpression (a) cerulean mRNA is robustly induced after a single heat shock in hs:col12a1a-p2a-Cerulean transgenic larvae. (b) col12a1a overexpression increases anti-Col XII immunoreactivity in developmental Col XII domains and in ectopic sites, including the spinal cord (arrow). Shown is a maximum intensity projection and a single optical section at the level of the spinal cord. Abbreviations: sc, spinal cord. (a-b) Views are lateral (dorsal is up, rostral is left) or transversal (section view in a; dorsal is up). BF: brightfield. Scale bars: 100 μm. |
|
Workflow for the quantitative analysis of immunohistochemistry or fluorescence in situ hybridization signals in a lesion site. A minimum intensity threshold is applied to the raw image (a-b), limiting pixels to only those of equal or higher intensity (c). After thresholding, a ROI is defined manually in which the thresholded pixel area is measured (d). The analysis is done without knowledge of the experimental condition. |