- Title
-
N-CoR is required for patterning the anterior-posterior axis of zebrafish hindbrain by actively repressing retinoid signaling
- Authors
- Xu, F., Li, K., Tian, M., Hu, P., Song, W., Chen, J., Gao, X., and Zhao, Q.
- Source
- Full text @ Mech. Dev.
|
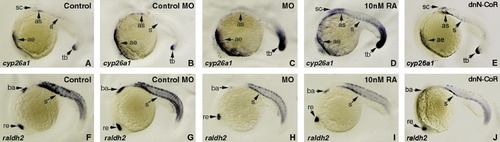
Knocking down n-cor resulting in elevation of endogenous RA signaling. Embryos at 1- to 2-cell stages are microinjected with n-cor MO (C and H) to knock-down n-cor expression or with dnN-CoR mRNA to reduce the function of N-CoR (E and J) and treated with 10 nM RA (D and I) to increase RA signaling. All treated embryos (C–E and H–J) together with wild-type control embryos (A and F) and control MO microinjected embryos (B and G) are grown to 20 hpf and examined for the expression of cyp26a1 (A–E) and aldh1a2 (also known as raldh2) (F–J), respectively, by whole mount in situ hybridization. All embryos are positioned anterior left and viewed laterally. cyp26a1 is mainly expressed at anterior epidermis, dorsal anterior spinal cord, somites and tail buds (A), while aldh1a2 is expressed at retina, branchial arches and somites (F) in the wild-type control embryo. Embryos microinjected with control MO display similar expression levels of cyp26a1 (B) and aldh1a2 (G) to the control embryos (A and F). Knocking down n-cor causes up-regulated expression of cyp26a1 in anterior epidermis and tail bud (C) and down-regulated expression of aldh1a2 in the regions of retina, branchial arches and somites (H). The expression changes also occur in the embryos treated with 10 nM RA (D and I) except that a strongly up-regulated expression of cyp26a1 in dorsal anterior spinal cord and somites in RA treated embryos (D). Overexpression of dnN-CoR causes up-regulated expression of cyp26a1 in dorsal anterior spinal cord and somites (E) and down-regulated expression of aldh1a2 in the regions of retina, branchial arches and somites (J). ae, anterior epidermis; as, anterior somites; ba, branchial arches; re, retina; s, somites; sc, dorsal anterior spinal cord; tb, tail bud. EXPRESSION / LABELING:
|
|
Knocking down n-cor posteriorizing anterior–posterior axis of zebrafish embryos in ways similar to increasing RA signaling. Embryos at 1- to 2-cell stages are microinjected with n-cor MO (C, C′ and G) to knock-down n-cor expression or treated with 50 nM RA (D and D′) or 10 nM RA (H) to increase RA signaling. All treated embryos (C, C′, D, D′, G and H) together with wild-type control embryos (A, A′ and E) and the control MO microinjected embryos (B, B′ and F) are grown to 80–90% epiboly and examined for the expression of otx2 (A–D and A′–D′) and hoxb1b (E–H) by whole mount in situ hybridization. The embryos of (A)–(H) are positioned anterior top and viewed dorsally. The embryos of A′–D′ are the same ones of A–D but positioned anterior front, respectively. otx2 is expressed anteriorly (A and A′) whereas hoxb1b is expressed posteriorly in the embryos (E). Microinjection of control MO does not change the expression patterns (B, B′ and F). Knocking down n-cor causes anterior reduction of otx2 expression (C and C′) and anterior expansion of hoxb1b expression (G), respectively. Embryos treated with 10 nM RA (H) or 50 nM RA (D and D′) display expanded expression of hoxb1b (H) and reduced expression of otx2 (D and D′), respectively. EXPRESSION / LABELING:
PHENOTYPE:
|
|
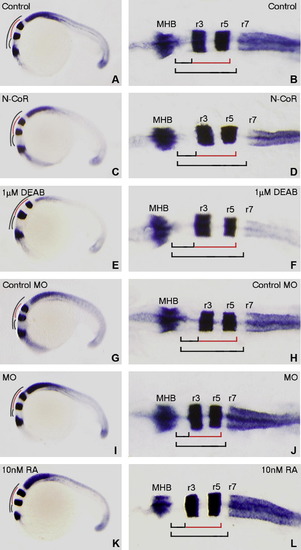
Alteration of N-CoR expression resulting in changes in r1–r6 hindbrain length that are similar to those caused by altering RA signaling in zebrafish embryos at 20 hpf. Embryos at 1- to 2-cell stages are microinjected with n-cor mRNA (C and D) or n-cor MO (I and J) to enhance or impair zebrafish N-CoR function, respectively. Alterations of RA signaling in embryos are performed by exogenous administration of 1 μM DEAB (E and F) or 10 nM RA (K and L) to reduce or enhance RA signaling, respectively. All treated embryos (C–F and I–L) together with wild-type control embryos (A and B) and control MO microinjected embryos (G and H) are grown to 20 hpf (22-somite stage) and then hybridized by RNA probes of engrailed2a (marking MHB), krox20 (marking r3 and r5) and hoxb4a (marking anterior boundary of r7). Whole mounted embryos (A, C, E, G, I and K) are positioned anterior left and viewed laterally whereas flat-mounted embryos (B, D, F, H, J and L) are positioned anterior left and viewed dorsally. The r1–r2, r3–r5 and r1–r6 lengths of the embryos overexpressing N-CoR (D) or treated with DEAB (F) are longer than those of the control embryos (B), respectively. The control MO microinjected embryos (H) have similar lengths of r1–r2, r3–r5 and r1–r6 to the control embryos (B). The lengths of r1–r2, r3–r5 and r1–r6 in the n-cor knocked down (J) or RA treated embryos (L) are shorter than those in the control embryos (B and H). MHB, midbrain–hindbrain boundary; r, rhombmere. EXPRESSION / LABELING:
PHENOTYPE:
|
|
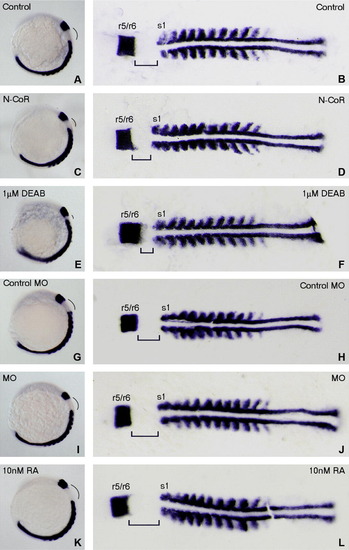
Alteration of N-CoR expression resulting in changes of hindbrain length between r6 and s1 similar to those changes caused by altering RA signaling in zebrafish embryos at 11-somites stage. Embryos at 1- to 2-cell stages are microinjected with N-CoR mRNA (C and D) or n-cor MO (I and J) to enhance or impair zebrafish N-CoR function, respectively. Alterations of RA signaling in embryos are performed by exogenous administration of 1 μM DEAB (E and F) or 10 nM RA (K and L) to reduce or enhance RA signaling, respectively. All treated embryos (C–F and I–L) together with wild-type control embryos (A and B) and control MO microinjected embryos (G and H) are grown to 11-somite stage and then hybridized by RNA probes of val (marking r5 and r6) and myoD (marking s1). Whole mounted embryos (A, C, E, G, I and K) are positioned anterior left and viewed laterally whereas flat-mounted embryos (B, D, F, H, J and L) are positioned anterior left and viewed dorsally. The lengths between r6 and s1 of the embryos overexpressing N-CoR (D) or treated with DEAB (F) are shorter than those of control embryos (B). The control MO microinjected embryo (H) has similar length between r6 and s1 to control embryos (B). The lengths between r6 and s1 of the n-cor knocked down (J) or RA treated embryos (L) are longer than those of the control embryos (B and H). r, rhombmere; s1, the first somite. EXPRESSION / LABELING:
PHENOTYPE:
|
|
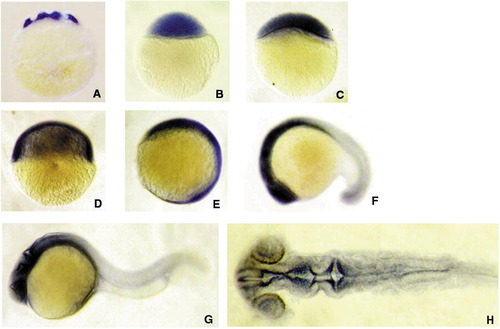
Whole mount in situ hybridization showing n-cor mRNA is widely present in zebrafish during early development. (A–E) animal pole top; (F–H) anterior left; (A–G) lateral view; (H) flat mount and dorsal view. A, 16-cell stage (1.5 hpf:); B, 512–cell stage (2.75 hpf); C, 30% epiboly (4.7 hpf); D, 50% epiboly (5.3 hpf); E, bud stage (10 hpf); F, 18-somite stage (18 hpf); G and H, 24 hpf. N-CoR mRNA message is widely distributed in early developmental stages from cleavage stage through gastrulation stage (A–D). At bud stage, the expression of n-cor is richer in anterior part than in posterior part (E). From 18 hpf to 24 hpf, n-cor message is mainly expressed in head region with very low expression in the other parts (F–H). Flat mount of 24 hpf embryos shows the expression is mainly in ventricles of forebrain, midbrain and hindbrain, and also in retina (H). |
Reprinted from Mechanisms of Development, 126(10), Xu, F., Li, K., Tian, M., Hu, P., Song, W., Chen, J., Gao, X., and Zhao, Q., N-CoR is required for patterning the anterior-posterior axis of zebrafish hindbrain by actively repressing retinoid signaling, 771-780, Copyright (2009) with permission from Elsevier. Full text @ Mech. Dev.