- Title
-
Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon
- Authors
- Scholpp, S., Wolf, O., Brand, M., and Lumsden, A.
- Source
- Full text @ Development
|
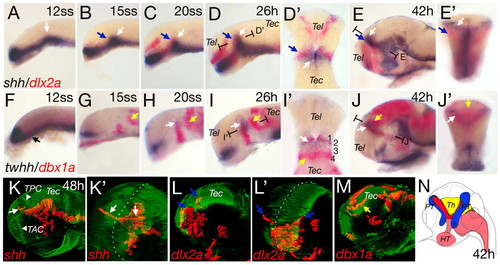
Anteroposterior differentiation in the mid-diencephalic territory (MDT). Whole-mount double in situ hybridization of wild-type embryos, with shh and dlx2a (A-E′) and twhh and dbx1a (F-J′). Section planes of D′,E′,I′,J′ are indicated. (A) shh expression in the ventral neural tube with the position of the presumptive ZLI indicated (white arrow). At the 15-somite stage, dlx2a expression starts in the forebrain (B,C; blue arrows). Horizontal section of D shows the abutting expression domains of dlx2a and shh (D′). shh expression extends to the dorsal and dlx2a expression is located more laterally (E, blue arrow). A cross-section in (E′) shows the ZLI (white arrow) and the prethalami (blue arrow). Onset of twhh expression in the presumptive ZLI is contemporaneous with shh (H and I, white arrow). twhh expression marks the v-shaped structure of the ZLI at 42hpf (J,J′). At 12 somites, dbx1a expression is anteroventral (F,G). At 15 somites, dbx1a is expressed at the base of the future ZLI (G), extending dorsally in later stages (H-J) and co-localized with twhh [horizontal section at 26 hpf (I′) and cross-section at 42 hpf (J′)]. From 15 somites onwards, a posterior domain of dbx1a can be detected, marking the presumptive thalamus (G-J, yellow arrows). At 42 hpf, the dbx1a-positive thalamus lies in a medial position, posterior to the ZLI (cross-section in J′). (K-M) Lateral and pseudo-frontal views of 3D reconstructions of confocal stacks of in situ hybridization combined with anti-acetylated tubulin staining. Broken white lines indicate the midline (K′ and L′). At 48 hpf, shh is expressed in the ZLI in two prongs (K,K′, white arrows). dlx2a expression in the prethalami is located ventral and lateral to the ZLI (L,L′, blue arrows). dbx1a is expressed posterior and dorsal to the shh expression domain (M, yellow arrow). (N) Scheme of the subdomains of the MDT based on marker expression at 42 hours: red, ZLI; blue, prethalami; yellow, thalamus; pink, shh-positive basal plate. HT, hypothalamus; PT, prethalamus; Th, thalamus; TAC, tract of the anterior commissure; Tec, tectum; Tel, telencephalon; TPC, tract of the posterior commissure. Embryos are oriented dorsal side to the top and anterior towards the left in all figures, except when indicated. EXPRESSION / LABELING:
|
|
Mis-expression of shh results in an increase of the MDT. shh mRNA (150 pg) was injected into one of 32 cells to generate randomly distributed shh-positive cell clones, shown by an FITC-coupled lineage tracer (A-A''). Mis-expression of shh leads to expansion of ptc1 (B-C', bracket) and induces ptc1 positive clones at ectopic sites (arrows). dlx2a as well as lhx5 are expanded following shh mRNA mis-expression (D-G', bracket). dlx2a is induced anterior to the ZLI (white arrow), but not posterior (blue arrow). dbx1a and emx2 expression increases in size after shh mRNA mis-expression (H-K', brackets). EXPRESSION / LABELING:
|
|
The MDT is reduced in embryos deficient for Hh signalling. (A) Inhibition of Hh signalling by cyclopamine treatment (70 µm) from 10-somites to 30 hours leads to a strong reduction of dlx2a expression anterior to the ZLI and dbx1a posterior to the ZLI (B). ptc1 expression is not detectable in these embryos (B'). Weaker phenotype observed with treatment between 20 somites and 30 hours (C; dlx2a-positive prethalamus is marked by blue bracket and dbx1a by yellow bracket), although ptc1 expression is still undetectable (C'). After 24 hpf, inhibition of Hh signalling has no detected effect (D) compared with wild-type siblings (A). Asterisks indicate the ZLI (A-D). Analysis of the smu phenotype reveals Hh dependency for gene expression in the MDT (E-H'). dlx2a is not detectable in the prethalamus (E,E', blue arrow), lhx5 expression is downregulated (F,F', blue arrow), dbx1a is absent form the thalamus (G,G'; yellow arrow), in contrast to the ZLI (asterisk). Similarly, emx2 is downregulated (H,H'; yellow arrow). Tec, tectum; Teg, tegmentum; Tel, telencephalon. EXPRESSION / LABELING:
|
|
Shh and Twhh have both redundant and unique function during MDT differentiation. Wild-type embryos or syu mutant embryos were injected with twhh-MO (0.5 mM), twhh-mRNA or both shh- and twhh-mRNAs. In wild type, ptc1 is expressed in hypothalamus, ZLI and basal plate (A). The width of the expression of ptc1 anterior and posterior to the ZLI is around 16 cells (bracket). twhh morphants show an overall reduction of ptc1 expression and the width of ptc1 domain is reduced to 14 cell diameters (B, bracket). In the syu mutants, ptc1 is down-regulated (C), and its domain at the ZLI shrinks to 5 cell diameters (bracket). In syu mutants additionally knocked-down for twhh, ptc1 expression is strongly reduced at the ZLI (D). (E) dlx2a expression in wild-type embryo at 28 hours. twhh morphants show a reduction of dlx2a in the prethalamus (F). Similarly, dlx2a is reduced in syu mutant embryos and the anterior ventral domain is not detectable (G, arrowheads). dlx2a expression is absent in the shh/twhh mutant/knockdown embryos (H), as in smu mutant embryos (I). Mis-expression of twhh-mRNA leads increased dlx2a expression in the prethalamus (J, bracket) and can be further enhanced by synchronous mis-expression of shh-mRNA (150 pg) (K, bracket). twhh morphants show similar thalamic expression of dbx1a compared with wild-type siblings (L,M). By contrast, syu mutant embryos show no detectable dbx1a in the thalamus (N), whereas expression in the tegmentum (asterisks) and ZLI (black arrow) seem unchanged. In shh/twhh mutant/knockdown embryos dbx1a expression is absent from the thalamus (O). smu mutants phenocopy the shh/twhh mutant/knockdowns (P). Mis-expression of twhh leads to weak expansion of thalamic dbx1a expression (Q), whereas combined shh/twhh mis-expression leads to a strong increase in the anteroposterior direction (R). EXPRESSION / LABELING:
|
|
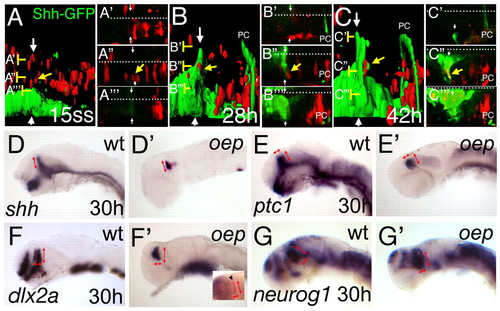
The basal plate is dispensable for ZLI formation and function. Rhodamine-dextran labelled cells (red) from a Shh-GFP transgenic embryo have been grafted into an embryo with the same genetic GFP-background (green) at sphere stage and further examined from 15 somites to 42 hpf (24 hours) on a confocal microscope. (A-C) Lateral views of the MDT of the same embryo at indicated stages after 3D reconstruction and surface rendering. (A'-A''', B'-B''', C'-C''') High magnification of the original dorsal sections of the scan at the indicated levels (brackets). White arrows indicate the ZLI and white dots indicate the midline. An indicated cell (yellow arrow) lies dorsal to the ZLI (A,A''). At 28 hpf, the cells are adjacent to the GFP positive ZLI (B,B'') and contribute to the ZLI at 42 hpf, shown by interdigitated red and green surfaces (C), and by a yellow overlay of the red rhodamine label with the GFP expression in the corresponding section (C''). PC marks the posterior commissure, marked by some shh-GFP positive axons. At 30 hours, oep mutant embryos were stained by indicated marker to analyse formation and function of the ZLI in embryos lacking the basal plate. In wild-type embryos, shh is expressed in the ventral neural tube as well as the ZLI (D). In oep mutants, shh can be detected only in the ZLI and the ventral hindbrain (D'). The dorsoventral extend of the ZLI is similar in wild-type and oep mutants (red arrows). ptc1 has a similar width to that of wild-type siblings in the diencephalon (E,E'; red arrows). dlx2a expression in the prethalamus has the same anteroposterior as well as dorsoventral extend in wild-type siblings as in oep mutants (F,F'; red arrows), as does neurog1 expression in the thalamus (G,G'; red arrows). Double in situ hybridization for the telencephalic marker emx1 in blue and dlx2a in red distinguishes the diencephalic dlx2a expression domain (inset in F', arrowhead). EXPRESSION / LABELING:
|
|
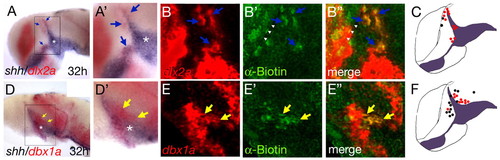
Direct Hh signalling is required for acquisition of proper MDT gene expression. Wild-type embryos were injected with a biotin-coupled lineage tracer and grafted into a smu mutant embryo at sphere stage. At 32 hpf, the embryos were fixed and stained for shh/dlx2a or shh/dbx1a by double in situ hybridization, followed by fluorescence detection of the biotin-containing wild-type cells. dlx2a-positive cell clones are detectable anterolateral to the ZLI (A, higher magnification in A'; blue arrows). A section of the same embryo shows co-localization between the red dlx2a with the green biotin (B-B'', blue arrows). dbx1a-positive cell clones are seen posterior to the ZLI (D, higher magnification in D'; yellow arrows). A section of the same embryo shows co-localization between the red dbx1a with the green biotin (E-E'', yellow arrows). (C,F) Summaries of all transplantation experiments showing dlx2a 14 wild-type clones, positive (12 red dots) and negative (2 black dots); and dbx1a 20 wild-type clones, positive (9 red dots) and negative (black dots, 7 anterior and 4 posterior to the ZLI). |
|
Overexpression of 150 pg shh-mRNA leads to an enlargement of the thalamic domain of neurog1 compared with wild type at 28 hpf (A,A¢, width indicated by yellow arrows). The thalamic expression of neurog1 is absent in smu mutant embryos at 32 hpf (B,B¢, yellow arrow marks the thalamus). In the presumptive ZLI, shh expression is detectable in oep mutant embryos at 20 somites first (C,C¢, marked by black arrow). EXPRESSION / LABELING:
|