- Title
-
BMP and non-canonical Wnt signaling are required for inhibition of secondary tail formation in zebrafish
- Authors
- Yang, Y., and Thorpe, C.
- Source
- Full text @ Development
|
Mesoderm tissues are mis-specified in the secondary tails of mfn. (A-J) Lateral view of expression of col2a (col2α; A,B), myoD (C,D), neurog1 (E,F), ntl (G,H) and tbx6 (I,J) in the posterior tail of wild-type and mfn zebrafish embryos. At 26 hpf, col2a (B), myoD (D) and ntl (H) are ectopically expressed in the secondary tail, but neurog1 is not (F). At 24 hpf, tbx6 (J) is also ectopically expressed in the secondary tail. Embryos in all images are mounted with anterior to the left. Dotted line in F indicates secondary tail. |
|
Secondary tail formation is independent of the role of BMP signaling in fate patterning. (A-E) Lateral view of zebrafish tails in wild-type and dorsalized embryos of indicated genotypes at 24 hpf, after whole-mount in situ for ntl. Primary and secondary tails are marked with arrowheads. C1-C4 refer to the degree of dorsalization using the dorso-anterior index. (F) Distribution of secondary tail phenotype in embryos following dorsomorphin (DM) treatment at the indicated time points. Secondary tails were scored by the presence of ectopic ntl expression. (G) Lateral view of the tail of a live embryo at 24 hpf, after DM treatment at 7 somites (7s). Note the secondary tail with fully developed ventral fin. (H-M) Dorsal view of embryos expressing gata1 (H,I), fli1 (J,K) at the 13-somite stage and myoD (L,M) at 24 hpf in the tail, after treatment with DMSO (H,J,L) and DM (I,K,M) at the 5-somite stage. (N,O) Dorsal view of myoD expression in the tail of wild-type (wt; J) and mfn (K) embryos at 22 hpf. Inset shows the lateral view. Expression of gata1 (I), fli1 (K) and myoD (M,O) is unaffected. EXPRESSION / LABELING:
PHENOTYPE:
|
|
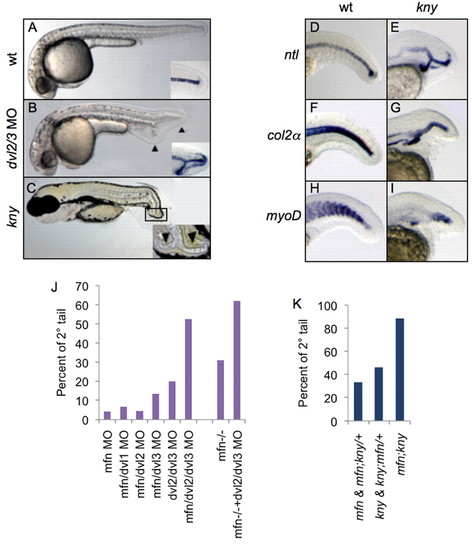
Non-canonical Wnt signaling functions with BMP to inhibit secondary tail formation. (A-C) Lateral view of wild-type (A, 24 hpf), dvl2/3 morphant (B, 24 hpf) and kny mutant (C, 72 hpf) zebrafish. Insets of A and B show ntl expression in the posterior tail. Inset of C shows a close-up of posterior tail. Primary and secondary tails are marked with arrowheads. (D-I) Lateral view of expression of ntl (D,E), col2a (col2α F,G) and myoD (H,I) in the tail of wild-type and kny embryos fixed at 24 hpf. ntl (E), col2a (G), and myoD (I) exhibit ectopic expression within the tails of kny. (J) Percentages of secondary tail formation in embryos injected with indicated combination of morpholinos (MO; 1 mg/ml each, except for ‘dvl2/dvl3 MO’ condition, where a concentration of 3 mg/ml for each MO was injected). For each column over 100 embryos from three separate experiments were scored. (K) Percentages of secondary tail formation in embryos from incross of mfn/+;kny/+ double mutant carriers. Embryos are categorized by phenotypes. Secondary tails in J and K were scored by ntl expression. EXPRESSION / LABELING:
PHENOTYPE:
|
|
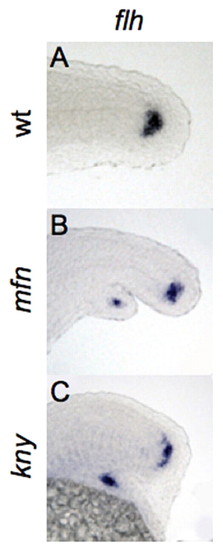
Bifurcation of the caudal end of notochord in BMP and non-canonical Wnt mutants. (A-C) Lateral view of expression of flh, marking the caudal end of notochord in wild-type, mfn and kny zebrafish embryos fixed at 24 hpf. flh is expressed in the secondary tail of mfn (B) and kny (C) mutants. |
|
N-cadherin is required to maintain tailbud integrity. (A,B) Percentages of bifurcated tail in zebrafish embryos injected with the indicated combination of morpholinos (MOs). For low concentrations, dvl2/3 (1 mg/ml each), mfn (0.5 mg/ml) and chd2 (0.1 mg/ml) MOs were injected. For each column over 100 embryos from three separate experiments were scored. (C-G) Lateral view of tails of embryos at 24 hpf injected with the indicated combination of morpholinos, after in situ hybridization for ntl. ntl expression in cdh2 and dvl2/3 MO morphants (C,D) is similar to wild type. Note that co-injection of a high concentration of chd2 and dvl2/3 MOs further enhanced the ntl expression from a single fork shaped pattern (E) to a multi-fork pattern (F), but co-injection of high concentration of chd2 and mfn MOs (G) did not result in a similar phenotype. This difference is consistent with the following antibody staining results (see below). For high concentrations, dvl2/3 (1.5 mg/ml each), mfn (1.5 mg/ml) and chd2 (0.25 mg/ml) MOs were injected. (H-J) Representative confocal microscopy images of tailbud in wild-type, kny and mfn embryos stained with pan-cadherin antibody at 21 somites; inset, close-up of the caudal end of notochord. Membrane staining pattern in the caudal end of notochord is shown in the wild type (H) and the mfn (J) mutant, whereas diffused staining pattern is shown in the kny mutant (I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
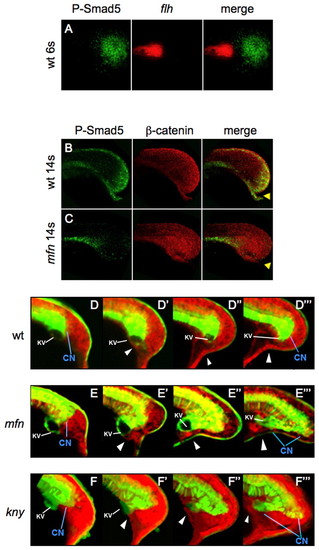
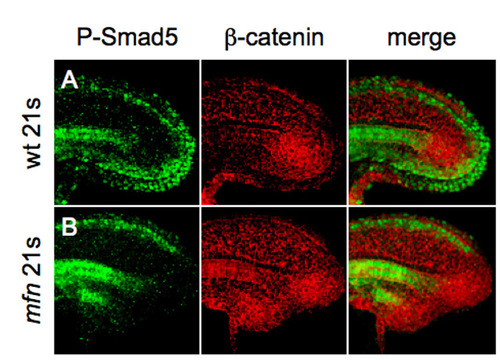
Defects of the caudal end of notochord in mfn and kny initiate during tailbud protrusion. (A) Dorsal view of flat-mounted tailbud of a 6-somite wild-type zebrafish embryo, after fluorescence in situ hybridization (FISH) for flh (red) and antibody staining for P-Samd5 (green); no colocalization (merge) is seen. (B,C) Lateral view of tails of 14-somite wild-type and mfn embryos exposed to P-Smad5 (green) and β-catenin (red) antibodies. P-Smad5 staining is absent in the posterior tailbud of mfn (C, merge). Arrowheads indicate the ventroposterior cells in the tailbud that are normally positive for P-Smad5 (B, merge). (D-F4) Confocal time-lapse recording of tailbuds in embryos expressing Tg(flh:EGFP) transgene (green) and mRFP (red) from the 10-somite stage. Shown are wild type (D-D4) and mfn (E-E4) and kny (F-F4) mutants. Note that transgenic EGFP is retained in the caudal notochord, floorplate and periphery of Kupffer′s vesicle (KV; marked by the white line). The caudal end of notochord (CN) is indicated by blue line. Arrowheads mark the ventroposterior cells in the tailbud. Embryos were all mounted laterally. EXPRESSION / LABELING:
PHENOTYPE:
|
|
BMP regulates posterior mesoderm cells of the tailbud to inhibit secondary tail formation. (A-J) All panels show the midline section of tails of Tg(flh:EGFP)-expressing zebrafish embryos (green) at the indicated time points after anti-Tbx6 (red) immunostaining. Tbx6-positive mesoderm cells in mfn embryos (B) shows detention in the posterior tailbud, when compared with wild-type embryos (A) at the same stage. A more wild-type-like movement of posterior mesoderm cells in kny embryos is shown in F and H. Note that eventually ectopic Tbx6-positive mesoderm cells are shown in the secondary tail domain of mfn (D) and kny (J) embryos. Arrowheads mark the ventroposterior mesoderm cells in the taillbud. All tails are flat mounted laterally. EXPRESSION / LABELING:
PHENOTYPE:
|
|
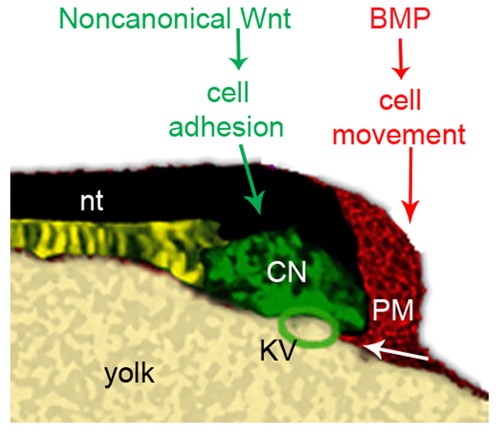
A model for the roles of BMP and non-canonical Wnt signaling in regulating tail development. Depiction of the tailbud of a 10-somite wild-type zebrafish embryo. During tailbud protrusion, BMP signaling in the posterior tailbud plays a crucial role in guiding the ventroposterior mesoderm cells to move beneath the Kupffer′s vesicle properly. Non-canonical Wnt signaling promotes intercellular adhesion of cells in the caudal end of notochord, allowing them to maintain cohesion during tail elongation. CNH, the caudal end of notochord; nt: notochord; PM, posterior mesoderm; KV, Kupffer′s vesicle. |
|
Ectopic expression of the CNH marker fgf4. (A-C) Ectopic expression of the CNH marker fgf4 in the secondary tail of mfn embryo (B) and dvl2/3 morphant (C) fixed at 24 hpf. Lateral view with anterior to the left. |
|
Distribution of morphological phenotypes of flh-expressing CNHs in mfn, kny and snh embryos fixed at the indicated time points. Right panel shows the lateral view of representative embryo in each phenotypic class. For each time point around 40 embryos were scored. |
|
Notochord bifurcation in kny embryos. (A-E) Lateral view of expression of ntl (A,B) and col2a (col2&alpha& C-E) in the tail of wild-type and kny embryos fixed at 24 hpf. Note the three separate flh-expressing CNH domains in B, triple-branched notochord (nt) in D and birfurcated notochord with detached hypochord (hc) in E. Each region is indicated by arrowheads. |
|
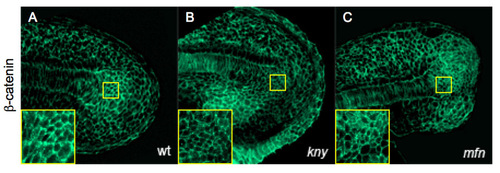
β-Catenin localization is unaffected in mfn and kny mutants. (A-C) Confocal microscopy images of tailbud in wild-type, kny and mfn embryos labeled with β-catenin antibody at 21 somites; inset, close-up of CNH region. Lateral view with anterior to the left. |
|
BMP signaling is active in the ventral-posterior tailbud. (A,B) Confocal microscopy images of tailbud in wild-type and mfn embryos labeled with β-catenin (red) and P-Smad5 (green) antibody at 21 somites. Note that in wild-type embryo (A), P-Smad5 staining is expressed in the posterior tailbud and extends under the notochord, whereas it is absent in mfn (B). |