- Title
-
A Mechanistic Study of the Osteogenic Effect of Arecoline in an Osteoporosis Model: Inhibition of Iron Overload-Induced Osteogenesis by Promoting Heme Oxygenase-1 Expression
- Authors
- Jiang, Z., Deng, L., Xiang, G., Xu, X., Wang, Y.
- Source
- Full text @ Antioxidants (Basel)
|
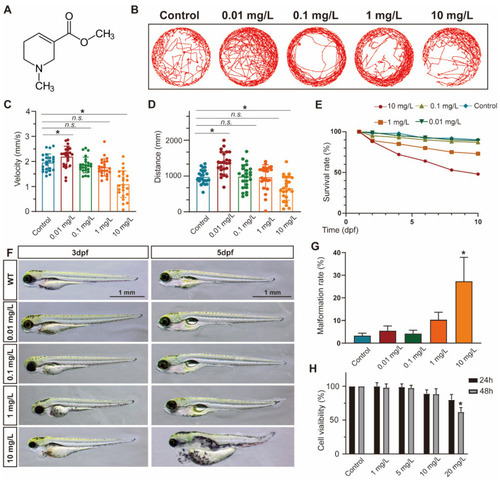
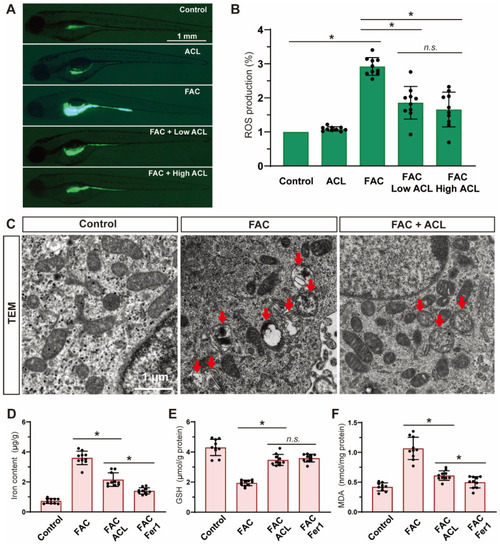
ACL exposure caused developmental malformations in zebrafish at high concentrations. ( PHENOTYPE:
|
|
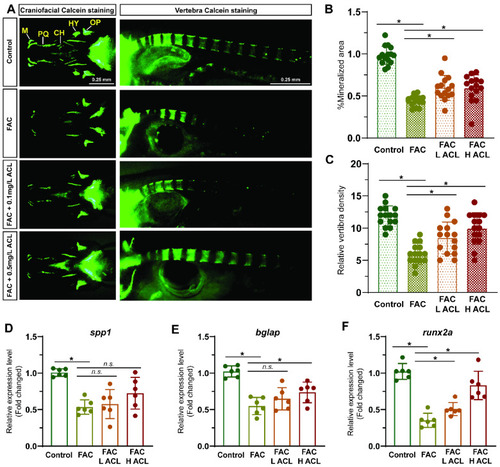
ACL attenuated the inhibition effect of FAC in bone formation in zebrafish. ( |
|
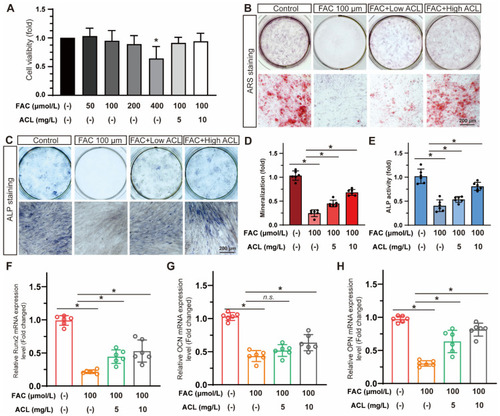
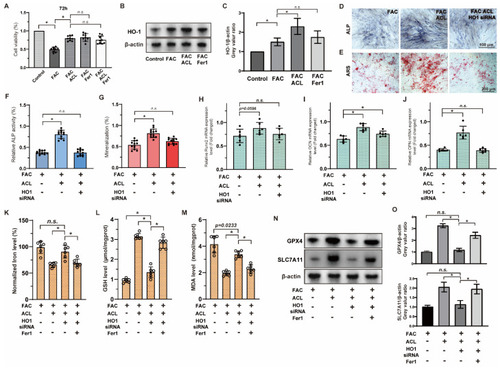
ACL promoted the osteogenic differentiation of MC3T3-E1 cells under FAC treatment. ( |
|
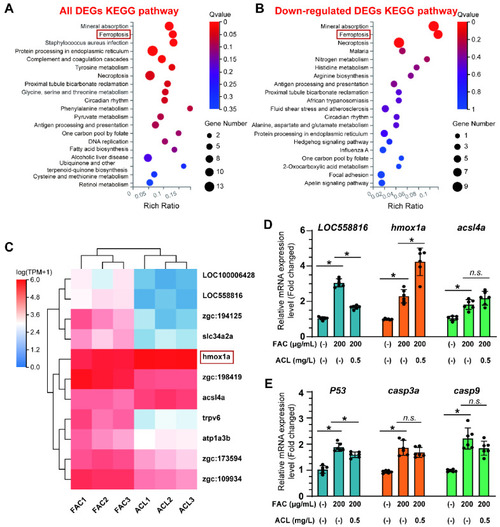
RNA-seq identified that ferroptosis may be involved in anti-osteoporosis effect of ACL under FAC exposure. ( |
|
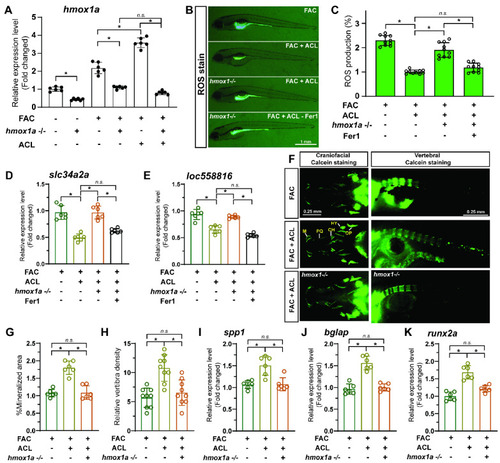
Ferroptosis was inhibited by ACL treatment under FAC exposure. ( PHENOTYPE:
|
|
Inhibition of ferroptosis by ACL can be reversed by HO-1 inhibition. ( |
|
The protective effect of ACL can be inhibited by |
|
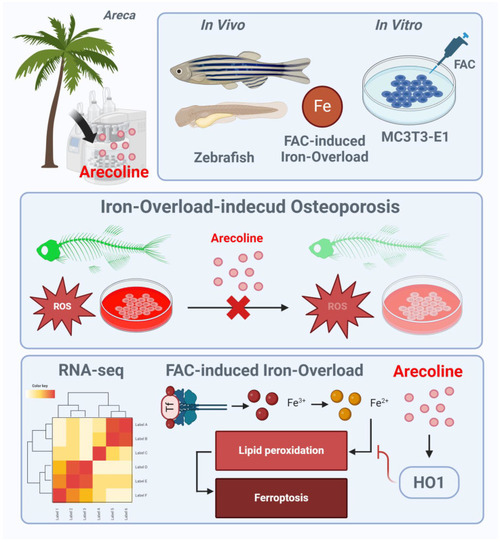
Schematic illustration of ACL for protecting FAC induced osteoporosis via upregulation of HO-1. FAC treatment leads to increased intracellular iron levels, lipid peroxidation, mitochondrial dysfunction, and oxidative stress, contributing to the inhibition of osteoblast differentiation and progression of osteoporosis. ACL attenuates these inhibitory effects by upregulating HO-1 expression. HO-1 activation promotes iron recycling and exerts antioxidant effects, thereby suppressing hallmarks of ferroptosis. The illustration figure was created by BioRender. |