- Title
-
Disruption of Epidermal Growth Factor Receptor but Not EGF Blocks Follicle Activation in Zebrafish Ovary
- Authors
- Song, Y., Chen, W., Zhu, B., Ge, W.
- Source
- Full text @ Front Cell Dev Biol

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
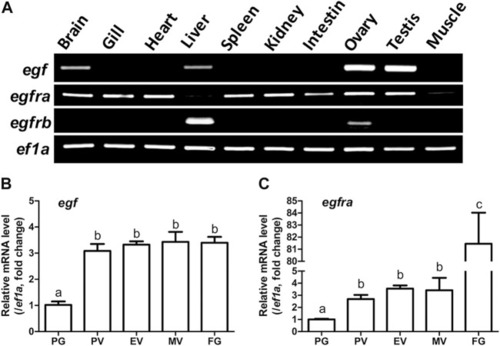
Tissue distribution of egf, egfra, and egfrb, and temporal expression profiles of egf and egfra during folliculogenesis. (A) RT-PCR analysis showed that egf was mainly expressed in the ovary and testis with weak expression in the brain, liver, and gill, whereas egfra was expressed in all organs, but with low levels in the liver and muscle. In contrast, egfrb was exclusively expressed in the liver and ovary with higher level in the liver. (B,C) The expression of egf and egfra mRNA was low in the PG follicles and increased significantly in the PV follicles. The mRNA level of egf maintained relatively constant after PV stage, whereas the expression of egfra showed a second surge in FG follicles prior to maturation. The expression levels of target genes were normalized to that of housekeeping gene ef1a, and expressed as fold change compared with that in PG follicles. Different letters indicate statistical significance (n = 3). PG, primary growth; PV, previtellogenic; EV, early vitellogenic; MV, mid-vitellogenic; FG, full-grown. EGF/egf, epidermal growth factor; EGFR/egfr, epidermal growth factor receptor. EXPRESSION / LABELING:
|
|
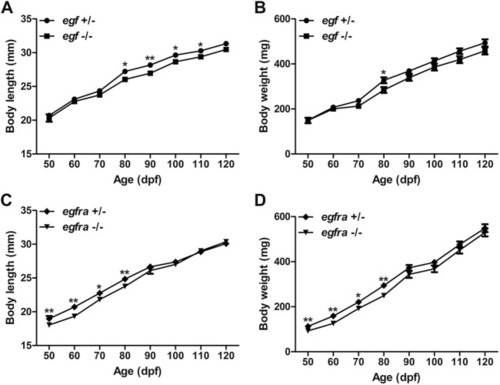
Growth performance of egf and egfra mutant males. (A,B) The standard body length and body weight of egf−/− fish were comparable with those of egf+/− fish with slight decrease after 70 dpf. (C,D) The standard body length and body weight of egfra−/− fish were slightly below those of egfra+/− fish from 50 to 80 dpf, and they became comparable after 90 dpf. *p < 0.05; **p < 0.01 (n = 19–50). PHENOTYPE:
|
|
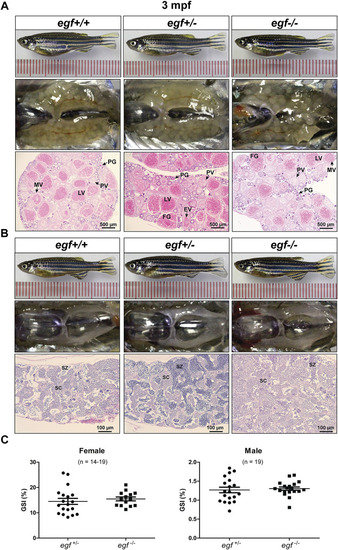
Gonadal development of egf mutant at 3 mpf. (A) Anatomical and histological examination of the ovary in egf mutant (egf−/−) and controls (egf+/+ and egf+/−). The egf-deficient follicles developed normally. (B) Anatomical and histological examination of the testis in egf mutant (egf−/−) and controls (egf+/+ and egf+/−). The spermiogenesis was normal in egf-deficient males. (C) GSI in females (n = 14–19) and males (n = 19). PG, primary growth; PV, previtellogenic; EV, early vitellogenic; MV, mid-vitellogenic; LV, late vitellogenic; FG, full-grown; SC, spermatocytes; SZ, spermatozoa. PHENOTYPE:
|
|
Reproductive performance of egf mutant at 4 and 7 mpf. (A) Egg numbers at 4 mpf in five fecundity tests (1st–5th). (B) Fertilization rates in five spawning tests at 4 mpf. (C) Egg number per spawning at 7 mpf. (D) Fertilization rate at 7 mpf. *p < 0.05; **p < 0.01 (n = 5 per test). PHENOTYPE:
|
|
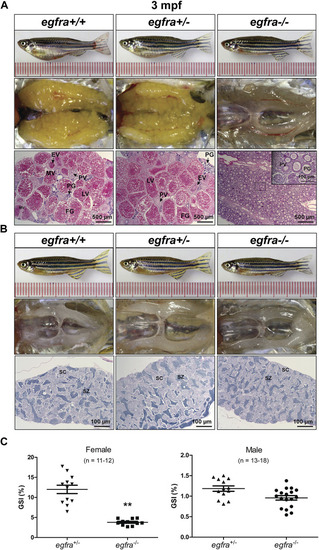
Gonadal development of egfra mutant at 3 mpf. (A) Anatomical and histological examination of the ovary in egfra mutant (egfra−/−) and controls (egfra+/+ and egfra+/−). The follicles of egfra-deficient females were arrested at PG stage with only a few entering early PV stage. (B) Anatomical and histological examination of the testis in egfra mutant (egfra−/−) and controls (egfra+/+ and egfra+/−). The spermiogenesis was normal in egfra mutant males. (C) GSI of egfra-deficient females (n = 11–12) and males (n = 13–18). The GSI of female mutant was much lower than that of control whereas no difference was found in males (**p < 0.01). PG, primary growth; PV, previtellogenic; EV, early vitellogenic; MV, mid-vitellogenic; LV, late vitellogenic; FG, full-grown; SC, spermatocytes; SZ, spermatozoa. PHENOTYPE:
|
|
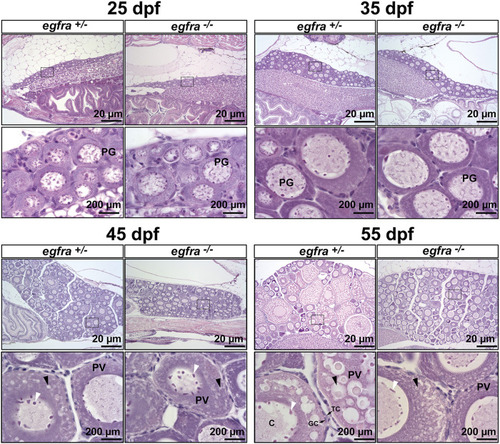
Effects of egfra deficiency on folliculogenesis in juvenile fish ovary. At 25 dpf, the presumptive perinucleolar oocytes at PG stage could be seen in both control (egfra+/−) and mutant fish (egfra−/−). These oocytes continued to grow in size in both genotypes at 35 dpf. At 45 dpf when puberty onset occurs in female zebrafish females, PV follicles with small cortical alveoli started to appear in the oocytes of both control (egfra+/−) and mutant fish (egfra−/−). However, the PV follicles in the control continued to grow with increasing number and size of the cortical alveoli as seen at 55 dpf, whereas the growth ceased completely in the mutant with some follicles containing rudimental cortical alveoli only. Six fish were sampled at each time point for each genotype. PG, primary growth; PV, previtellogenic; black arrow, cortical alveoli; white arrow, nucleoli at the periphery of the germinal vesicle; C, chromatin threads in the germinal vesicle; GC, granulosa cells; TC, theca cells. PHENOTYPE:
|
|
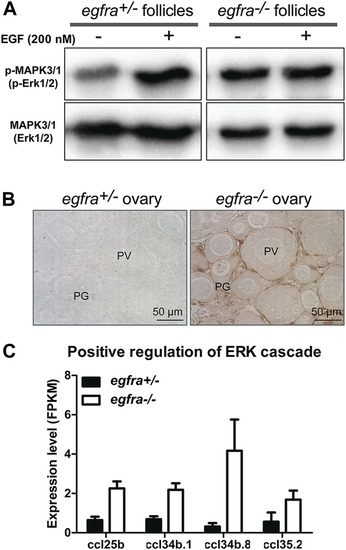
Loss of MAPK signaling response to EGF in egfra mutant ovary. (A) EGF treatment of ovarian fragments in vitro induced phosphorylation of MAPK3/1 (Erk1/2) in egfra+/− but not egfra−/− follicles. However, the mutant ovary showed higher basal level of MAPK phosphorylation despite its lack of response to EGF. (B) Immunohistochemical staining for MAPK3/1 phosphorylation in the control and mutant ovaries without EGF treatment. High level of phosphorylated MAPK3/1 was located in the somatic follicle cells of the egfra−/− ovary. (C) Increased expression of chemokine ligands (ccl25b, ccl34b.1, ccl34b.8, and ccl35.2) in egfra−/− follicles. EXPRESSION / LABELING:
PHENOTYPE:
|
|
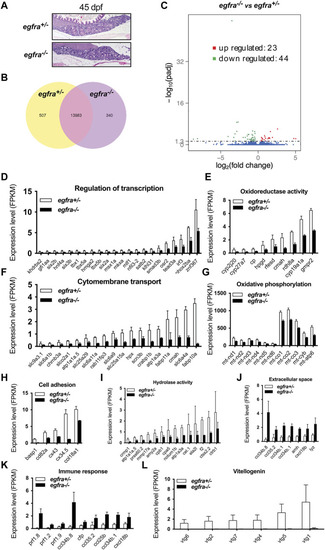
Transcriptome analysis for differentially expressed genes (DEG) in egfra−/− zebrafish ovary. (A) The ovaries of control (egfra+/−) and mutant (egfra−/−) at 45 dpf when follicle activation or PG-PV transition occurs. (B) Venn diagram illustrating DEGs in egfra+/− and egfra−/− ovaries. FPKM was used to normalize gene expression levels with FPKM >1 being the expression threshold. In total, 13,983 genes were expressed in the ovaries of both egfra+/− and egfra−/− fish, whereas 507 and 340 genes were only expressed in the ovaries of egfra+/− and egfra−/− fish, respectively. (C) MA plot showing the significantly downregulated (in green) and upregulated (in red) genes. (D–I) Enrichment of downregulated genes in transcription regulation, oxidoreductase activity, cytomembrane transport, oxidative phosphorylation (mitochondrial metabolic pathway), cell adhesion, and hydrolase activity. (J,K) Enrichment of upregulated genes in extracellular space and immune response. (L) Loss of expression of vitellogenin genes (vtg1, vtg2, vtg4, vtg5, vtg6, and vtg7) in mutant ovary follicles (egfra−/−). FPKM, fragments per kilobase million. |
|
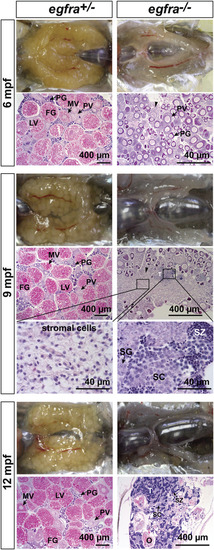
Progressive degeneration of follicles and sex reversal to males in egfra−/− females. Follicles in egfra−/− ovaries underwent progressive degeneration from 6 to 12 mpf. While the follicles were degenerating, the inter-follicular spaces were gradually occupied by somatic stromal cells, an early sign of masculinization. Testicular tissues with spermatogenic cells started to appear at 9 mpf and increased progressively afterward. By comparison, the ovaries of control egfra+/− females were filled with follicles of all developmental stages from PG to FG during the period. PG, primary growth; PV, previtellogenic; MV, mid-vitellogenic; LV, late vitellogenic; FG, full-grown; SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa; T, testis tissue; O, degenerating oocytes. PHENOTYPE:
|
|
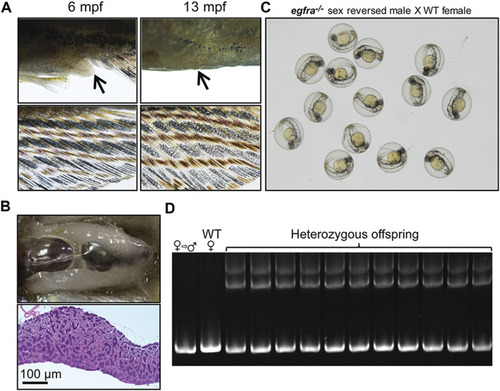
Development of secondary sexual characteristics during sex reversal and fertility test for sex-reversed males. (A) Protruding genital papilla (upper left, arrow) and a light anal fin (lower left) were observed in egfra−/− females at 6 mpf. The genital papilla disappeared and the anal fin turned golden in color in the same fish at 13 mpf. (B) The sex-reversed males (egfra−/−) showed normal testis and spermatogenesis at 13 mpf. (C) The sex-reversed males were fertile and could spawn with WT females to produce normal embryos. (D) All the offspring of sex-reversed males and WT females were heterozygous egfra+/− as genotyped by HMA. |
|
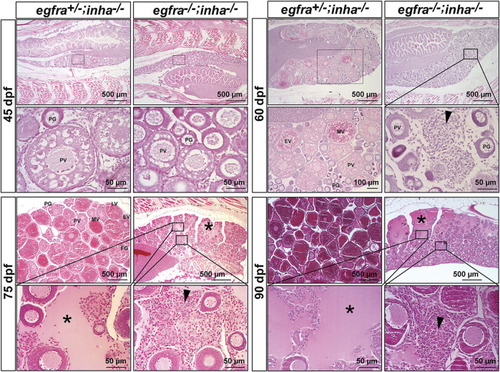
Double mutant of egfra and inha (egfra−/−; inha−/−) induced ovarian fibrosis and hydrovarium. The egfra+/− and inha−/- females were used as the control. Follicles were undergoing PG-PV transition at 45 dpf in both control and double mutant. These follicles continued to grow in the control ovary at 60 dpf with significant yolk accumulation in leading oocytes; however, the growth stopped completely at PG and early PV stages in the double mutant ovary (egfra−/−; inha−/−), which also showed fibrosis and accumulation of somatic stromal cells between follicles. The ovarian lamellae started to exhibit hydrovarium in the dorsal parts at 75 dpf in the double mutant, and the amount of fluid (asterisk) and fibrosis (arrowhead) continued to increase at 90 dpf. PG, primary growth; PV, previtellogenic; EV, early vitellogenic; MV, mid-vitellogenic; LV, late vitellogenic; FG, full-grown. PHENOTYPE:
|
|
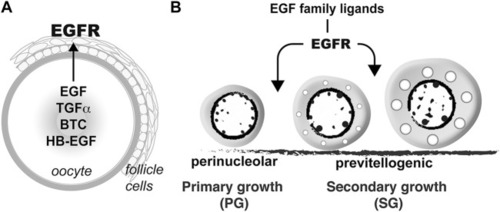
Hypothetical model on roles of EGFR signaling in controlling folliculogenesis in zebrafish. (A) Intrafollicular distribution of EGF family ligands and their receptor EGFR (Egfra) in the follicle (Tse and Ge, 2010), suggesting an oocyte-to-follicle cell signaling pathway. (B) EGF ligands-EGFR signaling pathway plays an important role in controlling early follicle development in zebrafish especially follicle activation or PG-PV transition. |

Unillustrated author statements PHENOTYPE:
|