- Title
-
Lnx2 ubiquitin ligase is essential for exocrine cell differentiation in the early zebrafish pancreas
- Authors
- Won, M., Ro, H., Dawid, I.B.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
lnx2a is expressed in the ventral pancreas during early pancreas specification. Lateral views (A and I), dorsal views (B–E), and ventral views (F–H). (A) lnx2a transcripts (blue) are detected in regions of the endoderm (arrow), forebrain (arrowhead), and the spinal cord at 24 hpf. Expression of pdx1 (B, D, and G), ins (C, E, and I) and foxa3 (F) are in red. lnx2a expression is observed in the antero-ventral part of pdx1-positive pancreas precursors at 21 (B) and 26 (D) hpf, but excluded from β-cells (ins+) in the dorsal pancreas at 21 (C), 26 (E), and 48 (I) hpf. At 48 hpf, lnx2a expression is detected in the ventral pancreas but not in the intestine, swim bladder, or liver (F–H). SB, swim bladder; In, intestine; Li, liver; Pa, pancreas; VP, ventral pancreas. (Scale bars: 50 µm.) EXPRESSION / LABELING:
|
|
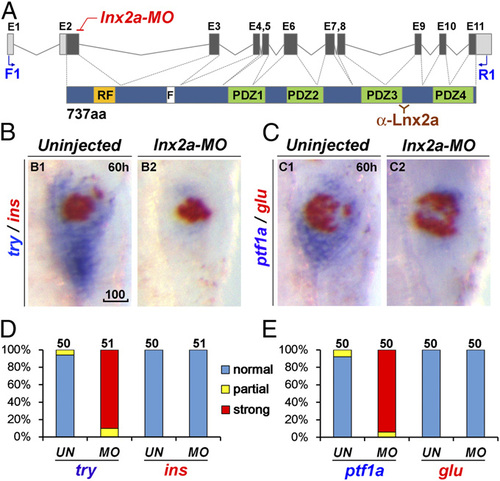
lnx2a knockdown causes defects in the exocrine pancreas. (A) Schematic drawing of the lnx2a locus containing 11 exons, and the Lnx2a protein (737 aa) containing a RING-finger domain (RF), the Numb binding NPAF motif (F), and four PDZ domains. The translation initiation site is located in exon 2, which encodes the RING-finger domain. The lnx2a-MO targets the splice donor site of exon 2. Primers in exon 1 (F1) and exon 11 (R1) are shown, as is the epitope for the Lnx2a antibody in the third PDZ domain. (B and C) The lnx2a-MO (5 ng) leads to inhibition of exocrine markers (try, ptf1a), but not endocrine markers (ins, glu), as seen by two-color WISH at 60 hpf. (D and E) Quantification of marker expression; effects were classified as strong, partial, and unaffected. MO, lnx2a-MO; UN, uninjected. (Scale bar: 100 µm.) |
|
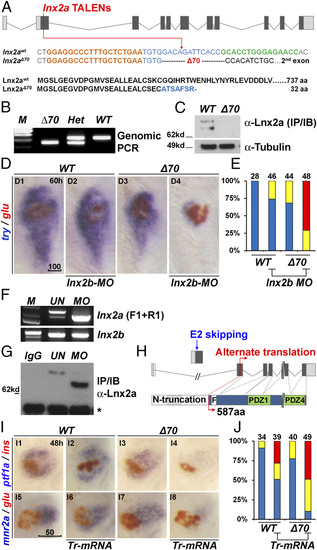
Generation of lnx2aΔ70 mutant using TALENs, and the functional redundancy of lnx2 genes. (A) Schematic representation of the lnx2a locus and the TALEN target site in the first protein coding exon (exon 2). TALEN targets (left, orange and right, green), and the lnx2aΔ70 mutation are shown below the drawing. Next, the protein sequences of WT and the lnx2aΔ70 frameshift allele are shown. Genotype of lnx2aΔ70 mutant was analyzed by the genomic PCR (B), and immunoblotting of endogenous Lnx2a protein (C). For validation of the anti-Lnx2a antibody, see SI Appendix, Fig. S2F. (D) Marker gene (try and glu) expression shows little effect in lnx2aΔ70 null mutants, and in lnx2b-MO–injected embryos at 60 hpf. However, lnx2b-MO injection into lnx2aΔ70 mutant embryos shows suppression of exocrine markers. (E) Quantification of pancreatic defects by analysis of trypsin expression, classified as in SI Appendix, Fig. S3 D and E for effectiveness of lnx2b-MO. (F–H) lnx2a-MO leads to production of N-truncated Lnx2a protein. (F) RT-PCR using primers F1 and R1 (defined in Fig. 2A) and 48 hpf embryo RNA, followed by sequencing, shows that lnx2a-MO injection results in exon 2 skipping and stabilization of the resulting transcript. lnx2b expression was not changed by lnx2a-MO injection. (G) Endogenous Lnx2a showed the expected size in controls, but a smaller protein was seen in lnx2a-MO–injected embryos, which was identified by mass spectrometry (SI Appendix, Fig. S4) as an N-truncated protein (587 aa) arising from an alternate start site in exon 3. (H) Schematic drawing of exon 2 skipping, alternate translation start site, and N-truncated protein in lnx2a-MO–injected embryos. (I) N-truncated Lnx2a (exon 2-skipped, as shown in F–H) has interfering effect. Tr-mRNA was injected into the WT and lnx2aΔ70 mutant embryos and analyzed at 48 hpf. (J) Quantification of pancreatic defects in I by analysis of ptf1a expression. Injection of Tr-mRNA into WT embryos has little effect, but leads to the exocrine pancreas phenotype in lnx2aΔ70 mutants. (Scale bars: D, 100 µm; I, 50 µm.) |
|
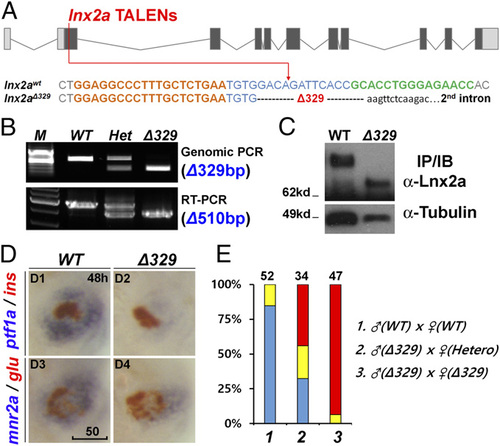
The lnx2aΔ329 mutant recapitulates the morphant phenotype. (A) Sequence of the lnx2aΔ329 mutation (Fig. 3A). The lowercase sequence in the mutant indicates that it is in intron 2, with lnx2aΔ329 having lost the exon 2 splice donor site. (B and C) Genotyping of lnx2aΔ329 by genomic PCR (Δ329), RT-PCR (Δ510 equaling exon 2) (B), and by Western blotting of embryo extracts showing the presence of N-truncated protein (C). (D) Phenotypic analysis of lnx2aΔ329 mutants at 48 hpf. (Scale bar: 50 µm.) (E) The quantification of pancreatic defects by analysis of ptf1a expression, classified as in Fig. 2D. Development of the exocrine pancreas is specifically suppressed in lnx2aΔ329. |
|
Ventral pancreas defects in lnx2aΔ329 mutant and lnx2a-MO–injected embryos can be rescued by knockdown of Numb. (A) The translation blocking MO for Numb (5 ng) was injected into WT, lnx2aΔ329-, and lnx2a-MO–injected embryos, and pancreatic phenotype was examined at 60 hpf. (Scale bar: 50 µm.) (B) Quantification of pancreatic defects by analysis of ptf1a expression. Exocrine pancreas defects were substantially rescued by knockdown of Numb; numb-MO had little effect in WT embryos. (C) Immunoblotting of embryo extracts with Numb antibody shows the efficiency of the numb-MO. |
|
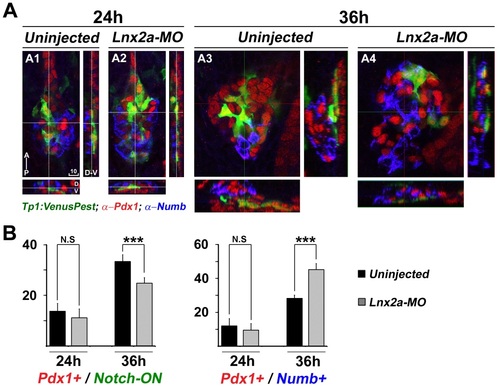
lnx2a-MO injection causes a reduction of Notch-responsive cells in the pancreas. (A) Tg(Tp1:H2BmCherry); Tg(Tp1:VenusPest) embryos were injected with lnx2a-MO (A2), numb-MO (A3), or both (A4). At 48 hpf, embryos were stained with anti-Numb antibody and analyzed by confocal microscopy; Z projections are shown. (Scale bar: 10 µm.) (B) Total number of Notch-OFF (Tp1:H2BmCherry+) and Notch-ON (Tp1:H2BmCherry+ and Tp1:VenusPest+) cells, as well as Numb-positive cells, were counted in all Z sections of four embryos for each category. **P < 0.005; ***P < 0.002. |
|
lnx2a-MO injection results in impaired cell proliferation in the ventral pancreas. (A) Tg(ptf1a:EGFP) embryos were incubated in 10 mM BrdU from 46 to 47 hpf, fixed, stained with anti-BrdU and anti-Numb antibodies, and analyzed by confocal microscopy. For clarity, the same plane of the BrdU channel is shown separately in addition to the merged images. (Scale bar: 10 µm.) (B) The average number of ptf1:EGFP+, Numb+ and ptf1:EGFP+/BrdU+ double-positive cells in the pancreas of 10 uninjected and lnx2a-MO–injected embryos are shown (***P < 0.001). |
|
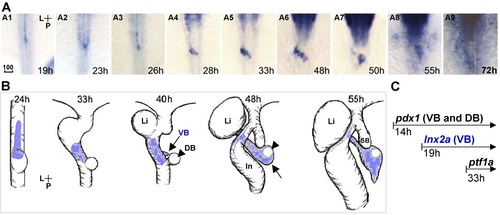
lnx2a is expressed in the ventral pancreas during early pancreas specification. Dorsal views (A and B). (A) lnx2a transcripts are detected in the ventral pancreas from 19 to 55 hpf; expression in nervous system is seen in rostral (upper) parts of the images. (B) Drawings of lnx2a expression domain (blue) during early pancreas development. Until 26 hpf (A1 - A3), lnx2a-expressing cells are located at the midline. After 24 hpf, the process of gut looping moves lnx2a-expressing cells to the left of the midline. Between 28 and 48h hpf, lnx2a-expressing cells are expanded to the right side and form a connection between the developing intestine and the dorsal bud (A4 - A6). After 48 hpf, lnx2a-expressing cells are mainly located in the surrounding region (arrow) of the primary islet which develops from the dorsal bud, (A6 - A8, arrowhead in B). (C) The earliest pancreas specific marker, pdx1, is expressed in all pancreas progenitors (VB and DB) from 14 hpf, the earliest ventral pancreas specific marker, ptf1a, is expressed from 33 hpf, and lnx2a is expressed only in the ventral pancreas from 19 hpf. P, posterior; L, Left; Li, Liver; VB, Ventral bud; DB, Dorsal bud; ln, Intestine; SB, Swim bladder; Scale bar, 100 µm. EXPRESSION / LABELING:
|
|
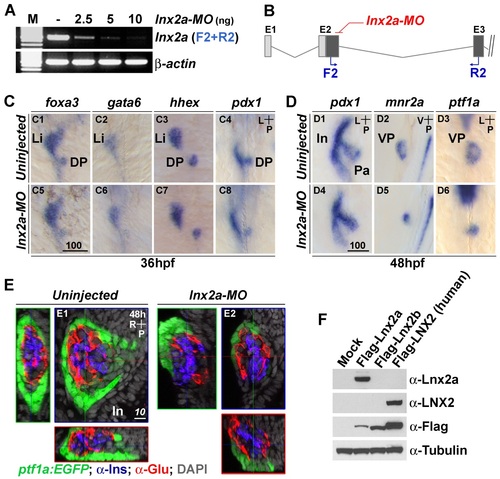
lnx2a knock-down affects development of the exocrine pancreas. (A) RT-PCR reveals that lnx2a-MO injection efficiently blocks pre-mRNA splicing between exon 2 and exon 3. (B) A schematic drawing indicates the location of primers in exon 2 (F2) and exon 3 (R2). (C and D) lnx2a is involved in the maintenance of ventral pancreas but not initial establishment of endoderm patterning. (C) At 36 hpf, foxa3, gata6, hhex and pdx1-positive cells develop normally in lnx2a-MO injected embryos. (D) At 48 hpf, pdx1-expressing pancreas precursor tissue is normally formed, but mnr2a and ptf1a-postive ventral pancreas precursors are reduced in lnx2a- MO injected embryos (D5 and D6). (E) Detection of lnx2a-MO effect using transgenic embryos and immuno staining. Tg(ptf1a:EGFP) embryos were injected with lnx2a-MO and stained for insulin (Ins) and glucagon (Glu) to visualize β-cells and α-cells, respectively. Confocal images at 48 hpf are shown along three axes. lnx2a-MO injected embryos display defective ventral pancreas (ptf1a: EGFP+), but normal dorsal pancreas-derived β-cells (Ins+) and α-cells (Glu+). (F) Western blot shows the specificity of the anti-Lnx2a polyclonal antibody. Flag-tagged zebrafish Lnx2a, Lnx2b and human LNX2 expression vectors were transfected into 293T cells, and lysates were analyzed by immuno blotting with anti-Lnx2a, LNX2, Flag and Tubulin antibodies, as indicated. Li, Liver; Pa, Pancreas; In, Intestine; DP, Dorsal pancreas; VP, Ventral pancreas; Scale bar, 100 µm (C5) and 10 µm (E1). |
|
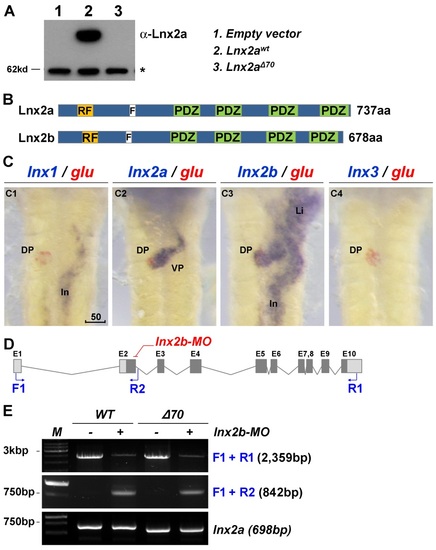
Comparison of lnx2a and lnx2b genes in pancreas development. (A) The lnx2aΔ70 mutant does not produce Lnx2a protein. 293T cells were transfected with empty vector or expression vectors for lnx2awt and lnx2aΔ70, and whole cell lysates were analyzed by immuno blotting with anti-Lnx2a antibody. (B) Domain structure of Lnx2a and Lnx2b. (C) Expression patterns of the lnx gene family in the endoderm, using glucagon for comparison, at 48 hpf. lnx2b is expressed in the whole endoderm, whereas lnx1 and lnx3 are not expressed in the pancreas. (D) Schematic illustration of the lnx2b locus and lnx2b-MO targeting site, the splice donor site of exon 2. The protein coding and non-coding exons are indicated as solid and open boxes, and primers in exon 1 (F1), intron 2 (R2), and exon 10 (R1) are shown. (E) As seen by RT-PCR at 48 hpf, injection of lnx2b-MO reduces full length mRNA and generates intron 2 read-through mRNA. Note that the lnx2b-MO does not induce exon skipping, unlike the lnx2a-MO. lnx2a expression levels were not changed in lnx2b-MO injected embryos in the WT or the lnx2aΔ70 mutant. DP, Dorsal pancreas; VP, Ventral pancreas; In, Intestine; *, Non-specific bands; Scale bar, 50 µm. EXPRESSION / LABELING:
|
|
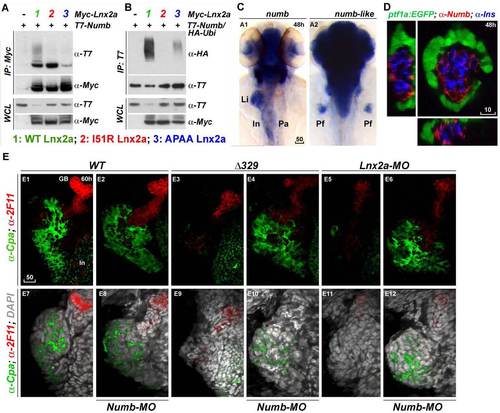
Numb is a target of Lnx2a in the zebrafish pancreas. (A and B) Lnx2a is an E3 ubiquitin ligase that mediates the ubiquitination and degradation of Numb (16, 17). Myc-tagged WT Lnx2a and I51R and APAA mutants were co-transfected into 293T cells with T7-tagged Numb (A) and HA-tagged ubiquitin (B). After 36 h, immunoprecipitation and immuno blotting were performed with the indicated antibodies. (A) Numb interacts with WT and I51R mutant, but not with APAA mutant Lnx2a. In particular, co-immunoprecipitated Numb with WT Lnx2a was highly modified. (B) poly-ubiquitination of Numb was induced by WT Lnx2a but not by the I51R or APAA mutant. (C) Expression pattern of numb during early pancreas development. Transcripts of numb were detected in the whole pancreas, liver and intestine at 48 hpf, whereas numb-like was found in the brain but not in the endoderm. (D) Immune-staining with Numb antibody in Tg(ptf1a:EGFP) embryos reveals that Numb is expressed in the primary islet but not in ptf1a:EGFP+ ventral pancreas. At 48 hpf, Tg(ptf1a:EGFP) embryos were stained with anti- Insulin and anti-Numb antibodies, and confocal images in three axes are shown. (E) Immuno staining shows that exocrine pancreas defects in lnx2aΔ329 mutants and lnx2a-MO injected embryos are rescued by the knock-down of Numb. After numb-MO injection into WT siblings, lnx2aΔ329 mutants, and lnx2a-MO injected embryos, embryos were stained with anticarboxypeptidase A (α-cpa) and ductal cell-specific antibody 2F11, at 60 hpf. Stained embryos were analyzed by confocal laser scanning microscopy, and Z projections are shown. lnx2aΔ329 mutants and lnx2a-MO injected embryos showed defects in Cpa+ exocrine pancreas that could be rescued by knock-down of Numb. Li, Liver; In, Intestine; Pa, Pancreas; Pf, Pectoral fin; GB, Gall bladder; Scale bar, 50 µm (A and E) and 10 µm (B). |
|
lnx2a-MO injection causes a reduction of Notch activity and induction of Numb accumulation at 36 hpf. (A) Tg(Tp1:VenusPest) embryos were injected with lnx2a-MO (A2 and A4). Embryos were stained with anti-Pdx1 and anti-Numb antibodies and analyzed by confocal microscopy; Confocal images at 24 and 36 hpf are shown along three axes. (B) Total number of Notch-ON (Tp1: VenusPest+) cells and Numb positive cells were counted in all Z sections of 5-10 embryos for each category. N.S, not significant; ***, P < 0.001; Scale bar, 10 µm. |
|
Lnx2a-MO injection affects both the specification and expansion of the ventral pancreas. Lnx2a-MO injection leads to inhibition of the ventral pancreas markers (ptf1a, mnr2a) from 36 hpf onward. HB, hindbrain; SC, spinal cord. |