- Title
-
The ascl1a and dlx genes have a regulatory role in the development of GABAergic interneurons in the zebrafish diencephalon
- Authors
- MacDonald, R.B., Pollack, J.N., Debiais-Thibaud, M., Heude, E., Talbot, J.C., and Ekker, M.
- Source
- Full text @ Dev. Biol.
|
The expression domains of the ascl1a, dlx, and gad1b genes overlap in the forebrain of the embryonic zebrafish. Single z sections on triple fluorescent in situ hybridizations. (A) In the forebrain lateral view of a WT embryo, the dlx1a and ascl1a are co-expressed throughout the telencephalon and prethalamus at 24 hpf, while ascl1b is not co-expressed at this stage. (B) The dlx1a, dlx5a, and gad1b genes are co-expressed in the telencephalon, prethalamus, and hypothalamus at 24 hpf. (C) The dlx1a, dlx5a, and gad1b genes continue to be co-expressed in the telencephalon and prethalamus at 48 hpf. The gad1b expression is increased in the dorsal tip of the telencephalon (arrow). The plane of section for (D) is shown as a dotted line in C′′′. (D) Cross-section showing the co-expression of dlx1a, dlx5a, and gad1b in the prethalamus (arrow). The rostral thalamus (arrowhead) is positive for gad1b expression but not dlx1 or dlx5a. Tel=telencephalon, Pth=prethalamus, Hy=hypothalamus, rTh=rostral thalamus. Scale bar in A–D=100 μm and in E=40 μm. |
|
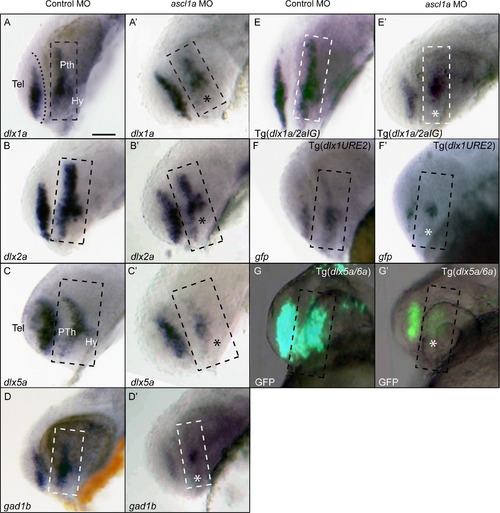
Ascl1a function is required for proper expression of the dlx and gad1b genes in the forebrain at 24 hpf. (A, B) Expression of dlx1a and dlx2a is reduced in the prethalamus and hypothalamus (asterisk), but not affected in the telencephalon of ascl1a morphants compared to control embryos. (C) The expression of dlx5a is particularly reduced in the prethalamus and hypothalamus in ascl1a morphants. (D) gad1b expression is unaffected in the telencephalon, but reduced in the prethalamus and hypothalamus. (E) Tg(dlx1a/2aIG:GFP) embryos reduced gfp mRNA expression in the prethalamus of ascl1a morphants but telencephalic expression appears unaffected. (F) Tg(dlx1URE2:GFP) embryos show reduced gfp mRNA expression in the telencephalon and prethalamus. (G) The loss of GFP expression in the Tg(dlx5a/6a:GFP) transgenic line is consistent with a loss of dlx5a expression. Dashed box represents prethalamus region. Tel, telencephalon; Pth, prethalamus; Hy, hypothalamus. Scale bar=50 μm. EXPRESSION / LABELING:
|
|
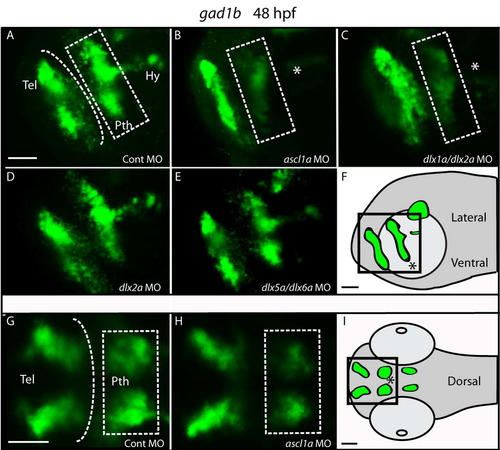
The expression of gad1b is impaired in the prethalamus but not in the telencephalon of ascl1a morphants and of dlx1a/2a double morphants at 48 hpf. Lateral (A–D) and dorsal views (A′–D′) of the forebrain of control and morphant embryos. (A) Expression of gad1b in embryos injected with the control morpholino is seen in the telencephalon (Tel), prethalamic (Pth) and hypothalamic (Hy) diencephalon. (B) Morpholino knockdown of ascl1a results in a loss of ventral prethalamic (dashed box) and hypothalamic (asterisk) gad1b expression. (C) Double morpholino knockdown of dlx1a/2a results in decreased prethalamic and hypothalamic gad1b expression. (D) In dlx5a/dlx6a morphants there is mild reduction of prethalamic and a decrease in hypothalamic gad1b expression similar to that observed in ascl1a and dlx1a/2a morphants. Dashed line indicates telencephalon-diencephalon boundary. Scale bar=50 μm. EXPRESSION / LABELING:
|
|
The expression of gad1b is impaired in the prethalamus but not in the telencephalon of ascl1a morphants and of dlx1a/2a double morphants at 48 hpf. Lateral (A–D) and dorsal views (A′–D′) of the forebrain of control and morphant embryos. (A) Expression of gad1b in embryos injected with the control morpholino is seen in the telencephalon (Tel), prethalamic (Pth) and hypothalamic (Hy) diencephalon. (B) Morpholino knockdown of ascl1a results in a loss of ventral prethalamic (dashed box) and hypothalamic (asterisk) gad1b expression. (C) Double morpholino knockdown of dlx1a/2a results in decreased prethalamic and hypothalamic gad1b expression. (D) In dlx5a/dlx6a morphants there is mild reduction of prethalamic and a decrease in hypothalamic gad1b expression similar to that observed in ascl1a and dlx1a/2a morphants. Dashed line indicates telencephalon-diencephalon boundary. Scale bar=50 μm. EXPRESSION / LABELING:
|
|
Exogenous dlx2a and dlx5a mRNA expression in ascl1a and dlx morphants rescues gad1b expression in the diencephalon (prethalamus and hypothalamus). Average number of embryos per injected treatment with normal (blue), and reduced (red) diencephalic gad1b expression. A clear difference in expression is observed between the embryos injected with the control MO+Tol2 mRNA (left insert) and the embryos injected with the ascl1aMO+Tol2 RNA (right insert). The latter is thereafter treated as the baseline for comparison with ascl1aMO+dlx2a (middle insert). Furthermore, exogenous expression of dlx5a, and particularly of dlx2a mRNA, is able to significantly decrease the proportion of embryos with reduced gad1b expression in the diencephalon of dlx1a/2a morphants. Data from 3 experimental replicates, with total individuals per treatment shown within graph ranging from n=144–173, p<0.05. |
|
Expression of dlx2a and dlx5a in the diencephalon of ascl1a morphants and expression of ascl1a in dlx1a/2a and dlx5a/6a double morphants. Whole mount in situ hybridization using probes for dlx2a (A, B) and dlx5a (C, D). Asterisks indicate prethalamic and hypothalamic reduction of dlx gene expression in 24 and 48 hpf morphant embryos. Tel, telencephalon, Pth, prethalamus, Hy, hypothalamus. Scale bar (A, C)=50 μm; scale bar (B, D)=100 μm. |
|
Diencephalic expression of gad1b is reduced in 48 hpf ascl1a morphants and in dlx1a/2a double morphants. Confocal images of fluorescent in situ hybridization in whole mount embryos for gad1b. (A) Control morpholino-injected embryos have normal telencephalic (Tel), prethalamic (Pth), and hypothalamic (Hy) expression of gad1b. (B) ascl1a morphants and (C) dlx1a/2a double morphants have decreased prethalamic and hypothalamic gad1b expression (asterisk). (D) Single dlx2a morphants do not show reduced gad1b expression. (E) dlx5a/dlx6a double morphants have decreased hypothalamic gad1b expression. (F) A diagram indicating the lateral field of view of confocal images in A–E. (G–H) Dorsal view of the forebrain in control morpholino injected fish and in ascl1a morphants (asterisk indicates a loss hypothalamic gad1b expression). (I) A diagram indicating the dorsal field of view of confocal images in G-H. Anterior is to the left. Scale bar=25 μm. |
|
Single knockdowns of dlx genes do not affect gad1b expression in the forebrain. (A) The wild type expression of the gad1b gene in the zebrafish forebrain at 48 hpf. The knockdown of dlx1a (B), dlx2a (C), dlx5a (E), or dlx6a (F) does not alter gad1b expression in the telencephalon (Tel), prethalamus (Pth) nor hypothalamus (Hy). (D) Expression of gad1b is not more severely reduced in the forebrain of embryos injected with MOs for dlx1a/2a/2b when compared to dlx1a/2a morphants (Fig. 3C). Scale bar=100 μm. |
|
Single knockdowns of dlx genes do not affect gad1b expression in the forebrain. (A) The wild type expression of the gad1b gene in the zebrafish forebrain at 48 hpf. The knockdown of dlx1a (B), dlx2a (C), dlx5a (E), or dlx6a (F) does not alter gad1b expression in the telencephalon (Tel), prethalamus (Pth) nor hypothalamus (Hy). (D) Expression of gad1b is not more severely reduced in the forebrain of embryos injected with MOs for dlx1a/2a/2b when compared to dlx1a/2a morphants (Fig. 3C). Scale bar=100 μm. |
|
Knockdown of dlx1a/2a or dlx5a/6a does not result in increased cell death in embryos at 24 hpf. (A) Control morpholino injected embryos show very little acridine orange staining at 24 hpf. (B, C) Injection with dlx1a/2a or dlx5a/6a morpholinos does not result in an increased labeling in the embryo, including the forebrain. (A′–C′) Magnified view of the head region of control and morphant embryos. |
Reprinted from Developmental Biology, 381(1), MacDonald, R.B., Pollack, J.N., Debiais-Thibaud, M., Heude, E., Talbot, J.C., and Ekker, M., The ascl1a and dlx genes have a regulatory role in the development of GABAergic interneurons in the zebrafish diencephalon, 276-85, Copyright (2013) with permission from Elsevier. Full text @ Dev. Biol.