- Title
-
Smarcd3b and Gata5 promote a cardiac progenitor fate in the zebrafish embryo
- Authors
- Lou, X., Deshwar, A.R., Crump, J.G., and Scott, I.C.
- Source
- Full text @ Development
|
Simultaneous inhibition of cBAF complex components leads to defects in myocardial specification. Suboptimal (1 ng of each MO in all cases) levels of morpholinos targeting smarcd3b, gata5 and tbx5 were injected as indicated. (A-H) RNA in situ hybridization for nkx2.5 expression at the eight-somite stage (13 hpf). Dorsal views with anterior towards the top of images. (I-P) RNA in situ hybridization for myl7 expression at 24 hpf. Dorsal views with anterior towards the bottom of images. (Q-X) Fluorescent images of 48 hpf hearts using the myocardial-specific myl7:EGFP transgene. Ventral views are shown with anterior towards the top of images. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Global overexpression of gata5/smarcd3b/tbx5 in zebrafish results in expanded expression of myocardial-specific genes. (A-C) Expression of nkx2.5 visualized by RNA in situ hybridization on 8-somite stage (13 hpf) wild-type (C), gata5-overexpressing (B) and cBAF-overexpressing (A) embryos. Dorsal views with anterior towards the top of images. (D-F) RNA in situ hybridization for myl7 expression at 24 hpf in wild-type (F), gata5-overexpressing (E) and cBAF-overexpressing (D) embryos. Dorsal views of the heart region are shown. (G-J) Light and fluorescent images of 24 hpf myl7:EGFP transgenic wild-type (I,J) and cBAF overexpressing (G,H) embryos. Lateral views with anterior towards the left are shown. EXPRESSION / LABELING:
|
|
Promotion of myocardial differentiation by gata5/smarcd3b in transplant experiments. (A,N) Schematics of experiments in which cells are transplanted to the host embryo margin (A) or animal pole (N) at sphere (Sp, 4 hpf) stage. (B-M) Transgenic myl7:EGFP donor cells from wild-type (E-G,K-M) or gata5/smarcd3b-overexpressing (B-D,H-J) donors were transplanted to the margin of wild-type host embryos. Rhodamine (red) indicates the lineage tracer in all donor-derived cells, EGFP indicates donor cells that have differentiated to form cardiomyocytes. (O-Z) Transplants were performed as above, with wild-type (R-T,X-Z) or gata5/smarcd3b-overexpressing (O-Q,U-W) donor cells placed in the animal pole of 4 hpf hosts. Embryos are 48 hpf, ventral (B-D) and lateral (E-Z) views with anterior towards the top. |
|
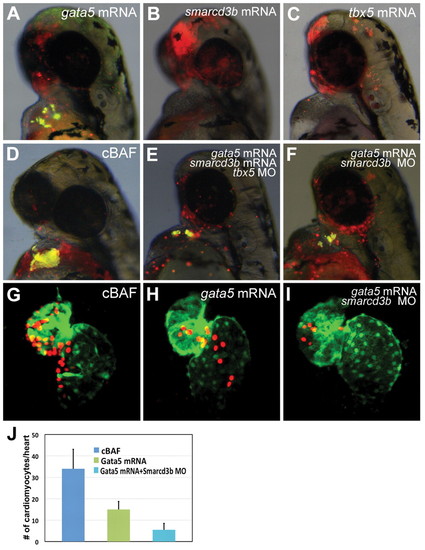
Role of single components of cBAF in promotion of cardiomyocyte differentiation. (A-F) Transgenic myl7:EGFP donor embryos were injected with tetramethylrhodamine dextran (red) and gata5 mRNA (A), smarcd3b mRNA (B), tbx5 mRNA (C), gata5/smarcd3b mRNA (D), gata5/smarcd3b mRNA and tbx5 morpholino (E), or gata5 mRNA and smarcd3b morpholino (F). At 4 hpf, donor cells were transplanted to the animal pole of wild-type hosts. Donor cell cardiomyocyte differentiation was assessed at 48 hpf by EGFP fluorescence. All images are overlays of red channel, green channel and bright field, such that donor cell-derived cardiomyocytes appear yellow. (G-J) Transgenic myl7:dsRedExp-nuc donor embryos were injected with gata5/smarcd3b RNA, gata5 RNA alone or gata5 RNA in conjunction with smarcd3b morpholino. Transplantation was carried out as above. At 48 hpf, donor-derived cardiomyocytes were quantified via nuclear-localized dsRed signal. (J) Results shown as number of donor cardiomyocytes per positive transplant embryo (mean±s.e.m., P<0.05). |
|
Cell-autonomous migration of gata5/smarcd3b-expressing cells to the heart. (A-E) Donor cells overexpressing gata5/smarcd3b (red) and wild-type cells marked by rhodamine green were co-transplanted to the animal pole of a wild-type 4 hpf host embryo (A). Boxed area in A is shown at higher magnification in the inset, showing that wild-type and cBAF donor cells are uniformly dispersed in the animal cap following transplantation. (F-H) Co-transplants where gata5/smarcd3b-expressing donor cells carry the myl7:EGFP transgene. Faint rhodamine green signal is evident in the head (arrowhead), whereas stronger EGFP is seen in the heart (arrow). (A) Dorsal view; (B,F-H) lateral views with anterior towards the top; (C) dorsal view onto head; (D,E) lateral views with anterior towards left. Asterisks in G denotes rhodamine-positive cells inside and adjacent to the heart that do not express EGFP. |
|
Properties of cBAF-overexpressing cells. (A-D) Series of still images from Movies 1 and 2 in the supplementary material showing cBAF-overexpressing cells (A,B) demonstrating a large number of fillopodia-like structures (arrowheads) when compared with wild-type cells (C,D). (E-P) Expression of ntl, nkx2.5, mespa and mespb visualized by RNA in situ hybridization at shield (6 hpf, E-G, K-P) and bud (10 hpf, H-J) stages in cBAF donor transplants, control (wild-type donor) transplants and wild-type (no transplantation) embryos. All images are animal cap views. |
|
Cells overexpressing gata5/smarcd3b form multiple cardiovascular lineages. (A,B) Donor kdrl:EGFP embryos were injected at the one-cell stage with gata5/smarcd3b RNA and tetramethylrhodamine dextran, and transplanted to the animal pole of wild-type host embryos at 4 hpf. Contribution to endocardium (arrowheads) and blood vessels neighboring the heart (arrows) was evident at 48 hpf. Lateral view of a 48 hpf embryo is shown, with the boxed region in A shown at higher magnification in B. (C,D) Donor embryos bearing no transgene were injected with gata5/smarcd3b RNA and tetramethylrhodamine dextran, and transplanted as in A,B. At 72 hpf, DAF-2DA staining was carried out. Arrowheads indicate the overlap of DAF-2DA staining (green) and tetramethylrhodamine dextran (red) in the outflow tract of the heart. Ventral view with anterior towards the top of images. (E,F) Donor Tg(tcf21:GVEcR;UAS:EGFP) embryos were injected with gata5/smarcd3b RNA and tetramethylrhodamine dextran and transplanted as in A,B. Contribution to ventricle (E, arrowhead) and head mesoderm/facial muscle (F, arrow) was evident at 48 hpf. (G,H) Heterochronic transplantation of 4 hpf donor cells from myl7:EGFP embryos injected with gata5/smarcd3b RNA and tetramethylrhodamine dextran to the head (G) and trunk (H) of 16 hpf host embryos. Combined bright-field/EGFP/rhodamine images are shown of transplant embryos at 48 hpf. Lateral views with anterior towards the top. |
|
Effects of host tissues and signaling pathways on the ability of cBAF-expressing cells to form myocardial cells. (A-R) Transgenic myl7:EGFP donor embryos were injected with tetramethylrhodamine dextran and gata5/smarcd3b RNA, and transplanted at 4 hpf into the animal pole of host embryos injected with morpholinos targeting sox32 (A-F), and gata5 and gata6 (G-I). (J-R) Donor cells as per above were transplanted into the animal pole of wild-type host embryos, with transplants treated from sphere stage (4 hpf) onwards with DMSO [as drug treatment control (J-L)], 40 μM SB-505124 (M-O) or 10 μM dorsomorphin (P-R). In A-F, cells were transplanted from the animal pole (A) to the host margin (M) or animal pole (A) as indicated. Embryos are shown at 48 hpf. (A-C,G-R) Lateral views with anterior towards the top of images; (D-F) ventral views with anterior towards the top. |
|
FGF signaling is required for the promotion of myocardial, but not endocardial, fate by the cBAF complex. (A-F) Transgenic myl7:EGFP donor embryos injected with gata5/smarcd3b RNA and tetramethylrhodamine dextran bearing (A-C) and not bearing (D-F) a hsp:dnfgfr1-EGFP transgene were transplanted to the animal pole of wild-type host embryos at 4 hpf, with heat-shock subsequently applied at 6 hpf. (G-L) Experiments were performed as in A-F, with donors carrying a kdrl:EGFP transgene in place of myl7:EGFP. All images are lateral views of 48 hpf embryos with anterior towards the top. |
|
Combinatorial knockdown of cBAF components does not prevent formation of endoderm and cranial/vascular mesoderm. (A-D) RNA in situ hybridization for expression of the endoderm-specific markers sox17 and fkd2 (foxa3) in triple gata5/smarcd3b/tbx5 morphants (sub-optimal doses of each as in Fig. 1) and control (wild-type) embryos. Embryos are at shield stage (6 hpf) lateral views with anterior towards the top of images (A,B) or at 24 hpf lateral views with anterior to left (C,D). (E,F) Cranial mesoderm of the jaw (marked with a tcf21:GVEcR;UAS:EGFP capsulin transgene) differentiates normally in triple gata5/smarcd3b/tbx5 morphants, but the show dismorphic jaw musculature. Transgene expression in the heart (arrowhead) is absent in morphants. (G.H) Vascular endothelial cell specification (as shown by a kdrl:EGFP transgene) is not affected in triple gata5/smarcd3b/tbx5 morphants. Boxed areas are shown at higher magnification in inset images, showing loss of kdrl:EGFP signal/endocardium in the cardiac region of morphant embryos. Lateral views of 48 hpf embryos are shown. |
|
cBAF promotes cardiomyocyte differentiation in zebrafish embryonic cell culture. (A-F) gata5/smarcd3b/tbx5 injected or wild-type (G-I) transgenic myl7:EGFP embryos were dissociated at 6 hpf and plated on laminin-coated glass coverslips. Cells were cultured for an additional 24 hours and imaged. Nuclei are indicated by DAPI staining. (D-F) Higher magnification view of cBAF overexpressing culture showing a cluster of EGFP-positive cardiomyocytes. Scale bars: 100 μm. |