- Title
-
A novel role for Glucocorticoid-Induced TNF Receptor Ligand (Gitrl) in early embryonic zebrafish development
- Authors
- Poulton, L.D., Nolan, K.F., Anastasaki, C., Waldmann, H., and Patton, E.E.
- Source
- Full text @ Int. J. Dev. Biol.
|
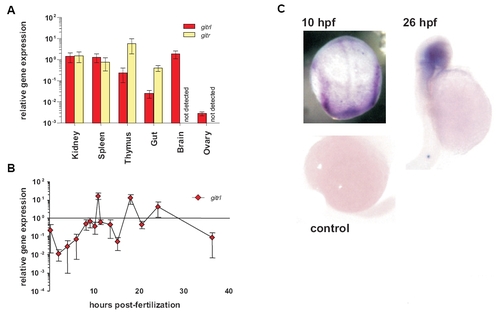
Expression of zebrafish gitrl and girt genes in adult tissues and during development. Real-time PCR was used to assess the expression of gitrl and girt in (A) adult zebrafish tissues and (B) at different time points during embryonic development. Gene expression was quantified using a standard curve method and is shown normalised to ef1α transcript levels and expressed relative to levels in adult kidney. Values are shown as mean ± SD of technical triplicates for an individual experiment representative of three biological replicates. In adult tissues girt mRNA was not detected in brain or ovary. In embryos, only gitrl is shown as no girt mRNA was detected during this period. (C) Localisation of gitrl transcripts at different time-points within developing embryos was investigated by in situ hybridization using a DIG-labelled antisense RNA probe. Distinct bands along the paraxial mesoderm can be seen at 10 hpf, with localization of expression to the head region by 26 hpf. A control embryo processed at 16 hpf in the absence of probe is shown to indicate the level of background staining and any yolk trapping. |
|
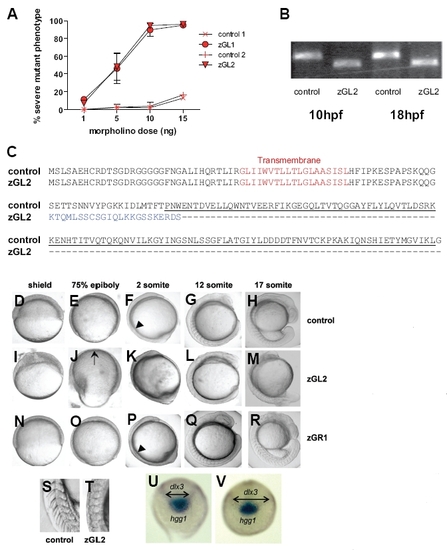
Morpholino oligonucleotide knock-down of gitrl during early development. (A) Embryos injected with increasing amounts of zGL1 (translation block), zGL2 (splicing block) or control MO were scored at 24 hpf for occurrence of a severe mutant phenotype defined by lack of clear somite boundaries and significant shortening of the embryo. The results are shown graphically as a mean percentage of total embryos injected ± SD of three experiments, with a minimum of 50 embryos injected per MO dose per experiment. Both zGL1 and zGL2 were equivalent in mediating the mutant effect which was not observed with control MOs. (B) The efficiency and persistence of the splice-blocking MO zGL2 were confirmed using RT-PCR. Following injection of 10 ng of zGL2, but not control MO, a smaller gitrl amplification product, diagnostic for the absence of exon 2, was obtained. The effect of zGL2 on splicing remained fully effective to at least 18 hpf. (C) To confirm the omission of exon 2, transcripts were cloned from zGL2 and control MO injected embryos and a total of 24 control and 48 zGL2 derived clones sequenced. All of the zGL2 derived clones displayed exon 1-3 splicing. This mis-splicing introduced a frameshift mutation such that the TNF family domain region, underlined, would be replaced by an alternative, tuncated C-terminus, indicated in blue. All of the control clones contained the full-length gitrl sequence. (D-R) Embryos injected with control, zGL2 and zGR1 MOs were compared from early gastrula through late segmentation stages. During gastrulation the blastoderm margin was poorly defined at the shield stage (I) in zGL2 morphants and did not advance to cover the whole of the yolk cell (J). Embryos tended to become mildly deformed during epiboly and the dorsal thickening and ventral thinning that is characteristic of late gastrulation was mispositioned in approximately 50 % of the embryos as indicated by the arrow in (J). In contrast both control (D,E) and zGR1 (N,O) MO-injected embryos remained phenotypically normal during gastrulation. The first somite boundary was clearly delineated in control (arrowhead in (F)) and zGR1 (arrowhead in (P)) but not in zGL2 morphant (K) embryos. Abnormal somite shape, optic primordium and head shape can be seen in (L). By late segmentation, zGL2 MO-injected embryos were significantly shorter with abnormal yolk extensions and abnormally-shaped somites (M). A magnified view of the posterior somites clearly shows the normal chevron shape in controls (S) and the abnormal shape and less-clearly defined boundaries in zGL2 morphants (T). (Equivalent phenotypes were also observed using the zGL1 and zGR2, data not shown). (U) Control or (V) gitrl morphants were probed at the tail-bud stage (10 hpf) using antisense RNA probes specific for hgg1 and dlx3. Double-headed arrows indicate the increased width of the dlx3 expression domain in gitrl morphants relative to control embryos. Data are representative of embryos from at least three independent experiments in which a minimum of thirty embryos per MO per experiment were injected. EXPRESSION / LABELING:
PHENOTYPE:
|
|
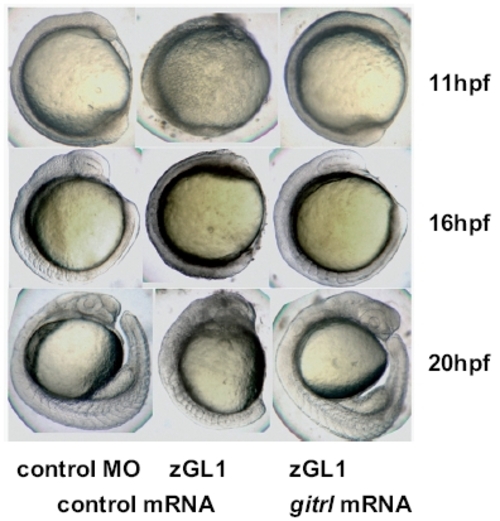
Rescue of the MO-induced gitrl morphant phenotype by co-injection of synthetic capped gitrl mRNA. Co-injection of synthetic capped mRNA and rescue of a normal phenotype was used to confirm that the MO-induced gitrl phenotype was specific and not due to off-target effects. Results with zGL1 (translation block) are shown, although equivalent rescue was also obtained for zGL2 (splice-block). The data are representative of embryos from at least three independent experiments in which a minimum of thirty embryos per MO per experiment were injected. |
|
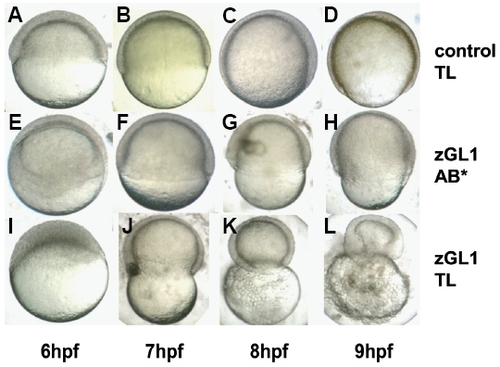
Strain-dependent penetrance of the MO-induced gitrl morphant phenotype. Embryos of the (A-D, I-L) TL and (E-H) AB* strains were injected with 10 ng of either (A-D) control 1 or (E-L) zGL1 MO. In zGL1-injected AB* embryos advancement of the blastoderm margin was delayed and incomplete (F-H), but these embryos successfully completed epiboly and survived through the segmentation period (see Fig. 3). In contrast, zGL1- injected TL embryos could not complete epiboly, and demonstrated a progressive contraction of the embryos around the yolk (J-L). PHENOTYPE:
|
|
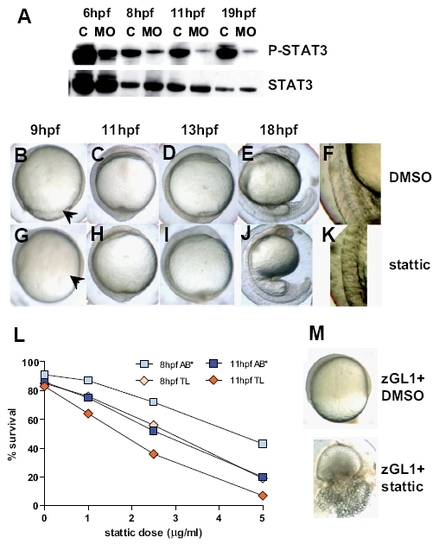
Identification of Stat3 phosphorylation as a possible mediator of Gitrl effects during embryogenesis. (A) Total protein lysates were generated from embryos injected with 10 ng of control1 (C) or zGL1 (MO) and harvested at 6, 8, 11 and 19 hpf. The lysates were analysed by western blotting using antibodies specific for phosphorylated (P-Stat3) and total (Stat3) Stat3 content. Embryos (AB*) treated with (B-F) DMSO or (G-K) 5 μM of stattic at 2-4 hpf were compared for phenotypic overlap with the gitrl morphant phenotype shown in figure 3. Stattic-treated embryos displayed incomplete blastoderm margin advancement (arrowhead in (G)) compared to controls (arrowhead in (B)), and indistinct somite boundary formation (H-K) similar to that seen in gitrl morphants. (L) TL embryos were more sensitive than AB* embryos to Stat3 inhibition. (M) TL embryos treated with a low dose of zGL1 (2.5 ng) were additionally treated with either DMSO or 2 mM of stattic at 2 hpf. Embryos that received low-dose MO plus DMSO were viable, but showed incomplete blastoderm margin advancement at 75 % epiboly, while embryos treated with low-dose zGL1 in addition to treatment with stattic showed a lethal phenotype. EXPRESSION / LABELING:
PHENOTYPE:
|
|
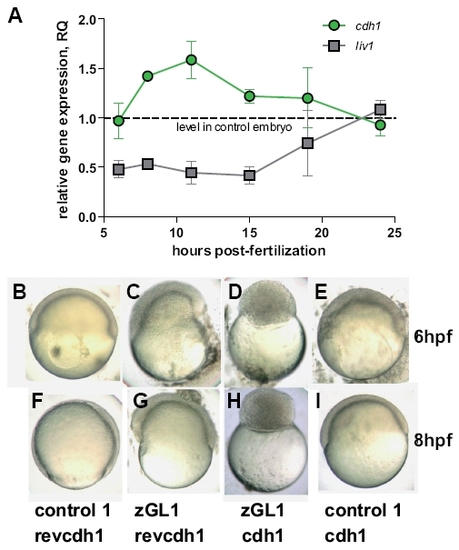
Gitrl and the Stat3-Liv1-Cdh1 axis. (A) Transcription of liv1 and cdh1 were assessed by real-time PCR at various time points during gastrulation and segmentation in embryos injected with 10 ng of either control1 or zGL1. Values were normalised to ef1α and levels in zGL1 injected embryos expressed relative to levels detected in control-injected embryos using the ΔΔCt method (ABI Prism 7700 Sequence Detection System, user bulletin no. 2). Mean values ± SD of three independent experiments are shown. Embryos injected with either (B,F) 10 ng of control 1 + 2.5 ng of cdh1 MO control (revcdh1), (C,G) 10 ng of zGL1 + 2.5 ng revcdh1, (D,H) 10 ng of zGL1 + 2.5 ng of cdh1 MO (cdh1) or (E,I) 10 ng of control 1 + 2.5 ng of cdh1 were observed at (B-E) 6 and (F-I) 8 hpf. Embryos treated with control MOs only were indistinguishable from wild-type embryos. Those treated with the zGL1 plus revcdh1 showed the characteristics of gitrl morphants previously described, and embryos injected with cdh1 MO plus control1 resembled the avalanche mutant (Kane et al., 1996). Surprisingly, embryos co-injected with both gitrl and cdh1 MOs had a novel phenotype characterized by arrest during the blastula period and a complete failure to enter epiboly. The phenotype shown in (D) and (H) persisted until the embryos died at approximately 15 hpf. EXPRESSION / LABELING:
PHENOTYPE:
|