- Title
-
Plakoglobin has both structural and signalling roles in zebrafish development
- Authors
- Martin, E.D., Moriarty, M.A., Byrnes, L., and Grealy, M.
- Source
- Full text @ Dev. Biol.
|
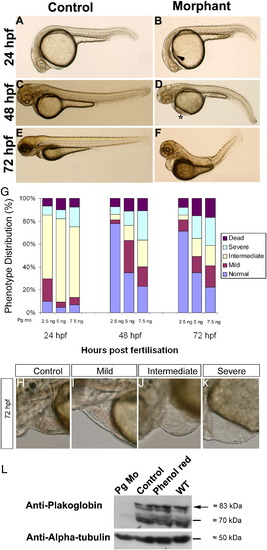
Effects of plakoglobin knockdown on zebrafish development. (A, C, E) Control 5 bp mismatch morpholino or (B, D, F) plakoglobin morpholino, 5 ng, were injected into embryos at 1- to 2-cell stage. Phenotypes were observed at (A and B) 24 hpf, (C and D) 48 hpf, and (E and F) 72 hpf. At 24 hpf, (B) morphant embryos displayed oedema (arrowhead), reduced heart size and a delay in midbrain–hindbrain boundary formation. At 48 hpf, (D) morphant embryos displayed cardiac oedema, reduced heart size, kinked tail, blood pooling (asterisk) compared to (C) controls. At 72 hpf, (F) morphant embryos displayed defects including cardiac oedema, blood pooling and a kinked tail. The numbers of embryos that displayed the above phenotypes were (A) 120/129 (B) 111/137 (C) 120/129 (D) 20/137 (E) 120/129 (F) 24/137. (G) Either 2.5 ng, 5 ng, or 7.5 ng, of plakoglobin morpholino was injected into embryos at 1- to 2-cell stage and the phenotypes scored at 24-, 48-, and 72-hpf. The percentage of embryos classed as normal (blue), mild (maroon), intermediate (yellow), severely affected (light blue) or dead (purple) for each dose at each timepoint are displayed. At 24 hpf, a similar percentage of embryos was affected at all doses. However, by 48 hpf, embryos injected with 2.5 ng appeared normal. 50 embryos were injected per experiment, n = 3 separate experiments. (H–K) Lateral views of 72 hpf plakoglobin morphant embryo hearts with anterior to left and dorsal to top. The cardiac phenotype ranged from a (H) normal phenotype similar to control injected embryos 49.15%, (I) mildly affected 16.62%, (J) intermediate 14.61% and (K) severely affected 19.65% morphant embryos alive at 72 hpf n = 129. (L) Western blot analysis of 72 hpf plakoglobin- or control-morpholino injected embryos, phenol red injected vehicle control and wildtype embryos using anti-plakoglobin antibody. Plakoglobin was detected in control morpholino injected embryos, phenol red and wildtype embryos (arrow) but not in morphant embryos confirming knockdown of the protein by the morpholino. An anti-α-tubulin antibody was used as a loading control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
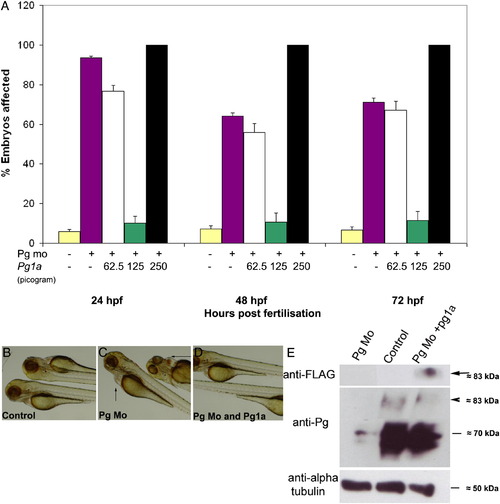
Plakoglobin-1a RNA rescues the morphant phenotype in a dose-dependent manner. (A) In each experiment, 5 ng of control morpholino (yellow) or plakoglobin morpholino was injected on its own (purple) or with 62.5 pg (white), 125 pg (green) or 250 pg (black) of plakoglobin-1a RNA. At 72 hpf, injection of plakoglobin morpholino alone resulted in 71% affected embryos while co-injection of 62.5 pg of morpholino and plakoglobin-1a resulted in 67% affected embryos. Co-injection of 125 pg of plakoglobin-1a resulted in only 11% of embryos exhibiting a morphant phenotype. Co-injection of 250 pg of plakoglobin-1a and plakoglobin morpholino resulted in all embryos showing either an abnormal phenotype or death. (B) Control morpholino, (C) plakoglobin morpholino, or (D) plakoglobin morpholino and 125 pg of plakoglobin-1a, were injected into embryos at 1- to 2-cell stage. At 72 hpf, the (C) morphant phenotype, including cardiac oedema (arrows), was rescued in (D) plakoglobin morpholino and plakoglobin-1a co-injected embryos. (E) Translation of injected plakoglobin-1a mRNA was confirmed by western blotting of 24 hpf embryo lysate with an anti-FLAG antibody. A band of approximately 83 kDa was detected corresponding to the expected size of FLAG tagged plakoglobin-1a (arrow). Blotting with an anti-plakoglobin antibody confirmed knockdown of plakoglobin in morphant embryos and partial rescue of expression in co-injected embryos (arrowhead). An anti-α-tubulin antibody was used as a loading control. PHENOTYPE:
|
|
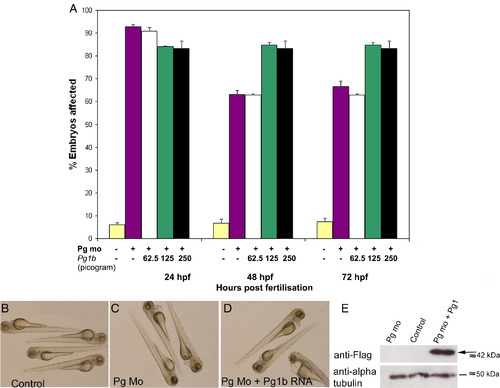
Plakoglobin-1b RNA fails to rescue morphant phenotype at three doses. (A) Phenotypic analysis of embryos injected with control morpholino (yellow), plakoglobin morpholino (purple), co-injection of morpholino and either 62.5 pg of plakoglobin-1b (white), 125 pg of plakoglobin-1b (green), or 250 pg of plakoglobin-1b (black). Co-injection of plakoglobin-1b failed to rescue morphant phenotype at any of the doses injected (P < 0.05, vs. control). In 125 pg and 250 pg doses, co-injection of plakoglobin-1b increased the number of embryos with an abnormal phenotype at 48- and 72-hpf (P < 0.05, vs. morphant). (B) Control injected embryos were unaffected and (C) plakoglobin morpholino injected embryos displayed the morphant phenotype. (D) Co-injection of morpholino and plakoglobin-1b RNA failed to rescue the morphant phenotype. (E) Western blot analysis of 24 hpf plakoglobin morpholino, control morpholino or plakoglobin morpholino and plakoglobin-1b co-injected embryos. An anti-FLAG antibody was used to detect FLAG-tagged plakoglobin-1b protein (arrow). An anti α-tubulin antibody was used as a loading control. The presence of FLAG protein confirms plakoglobin-1b was translated. |
|
Expression of cardiac marker genes in plakoglobin morphant and control morpholino injected embryos. (A and B) lateral views of 24 hpf and (C–J) ventral views of 48 hpf wholemount embryos. (A) Nkx2.5 at 24 hpf was expressed to the left of the midline in control injected embryos. (B) Nkx2.5 expression is reduced in morphant embryos but in the correct location. (C) Cmlc2 is expressed in both atrium and ventricle in 48 hpf control embryos and there is decreased expression in (D) morphant embryos. (E) Vmhc delineates ventricle specification at 48 hpf and (F) is decreased in morphant embryos. (G and I) Expression of both notch1b and bmp4 expression was restricted to the atrio-ventricular boundary (black arrows) in control morpholino injected embryo hearts. (H and J) In morphant embryos, both cardiac valve markers were expressed throughout the heart and were not restricted to the atrio-ventricular boundary (white arrows). The numbers of embryos with the displayed phenotype were (A) 62/65 (B) 55/63 (C) 72/75 (D) 53/72 (E) 68/70 (F) 40/72,(G) 77/80, (H) 67/85, (I) 74/78 and (J) 68/74. |
|
The structures of control and morphant cardiac valves were examined. (A) In control hearts, endothelial cells in the atrioventricular boundary have cuboidal morphology. (B) In morphant hearts, endothelial cells have a squamous morphology. (C) Higher magnification of the boxed area in (A). (D) Higher magnification of the boxed area in (B). Atria are indicated by (A) and ventricles by (V). The number of embryos with the displayed phenotype were (A) 2/2 and (B) 2/2. Scale bar is 10 μm for A and B and 2 μm for C and D. PHENOTYPE:
|
|
Reduced circulation in morphant embryos. (A, B) Lateral view of O-dianisidine stained 48 hpf (A) control and (B) morpholino injected embryos. Blood was present in the anterior blood vessels, the heart, the common cardinal vein, the dorsal aorta, and posterior cardinal vein. In the morphant embryo, blood was pooled as it exited the common cardinal vein and there was a reduction in blood cells in the anterior blood vessels. (C, D) Laser-scanning immunofluorescence microscopy images of transverse sections of 72 hpf embryo hearts. Fluorescence images with anti-E-cadherin antibody (red), a marker for epithelium and haematocytes, in (C) control morpholino injected embryo. Haematocyte numbers were reduced in (D) morphant heart. Hearts are outlined by white dotted lines. (E–H) The endothelial specific transgenic fli1:EGFP embryos injected with (G) control or (H) plakoglobin morpholino were analysed under light (E and F) and fluorescence microscopy (G and H) for vascular defects at 72 hpf. There was no alteration in vasculature in morphant embryos despite evident blood pooling (arrow). PHENOTYPE:
|
|
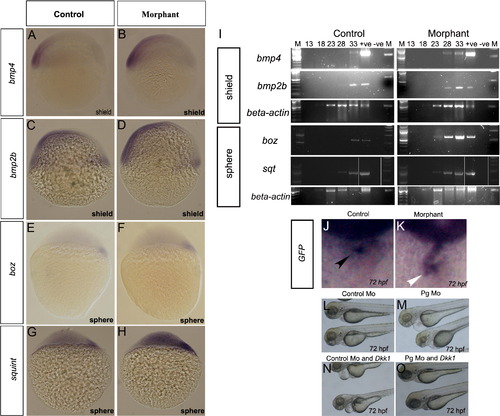
Loss of plakoglobin increases expression of signalling genes in early stage embryos. Plakoglobin morphant embryos have increased expression of (B) bmp4, (D) bmp2b, (F) bozozok and (H) sqt compared to their respective control injected embryos (A, C, E and G). The numbers of embryos with the above phenotype were (A) 49/53 (B) 47/55, (C) 85/91 (D) 54/87 (E) 50/55 (F) 38/62(G) 61/65 (H) 49/63. (I) Semi-quantitative RT-PCR amplification from shield and sphere stage plakoglobin and control morpholino injected embryos. Lane order: 100 bp ladder, PCR cycle 13, 18, 23, 28, 33, positive plasmid control, negative template control and 1 Kb ladder. β-actin was used as a template control. Expression of bmp4, bmp2b and squint were detected five cycles and bozozok was detected ten cycles earlier in morphants than control embryos. Analysis of GFP expression in morpholino injected TOPdGFP fish. (J) In control morpholino injected fish, GFP expression is detected at the atrioventricular boundary (black arrowhead). (K) In morphant embryos, GFP is expressed throughout the heart (white arrowhead). The number of embryos with the displayed phenotype were (J) 31/39 and (K) 34/43. Co-expression study of Dkk1 and control or plakoglobin morpholino. (L) Control morpholino injected embryos at 72 hpf with normal hearts (165/165). (M) Plakoglobin morphants at 72 hpf with abnormal hearts including oedema, reflux of blood between chambers (88/149). (N) Co-injection of 20 pg of DKK1 and control morpholino resulted in oedema and reflux of blood between chambers (30/128). (O) Co-injection of 20 pg of Dkk1 and plakoglobin morpholino with normal cardiac phenotypes (10/162). |
|
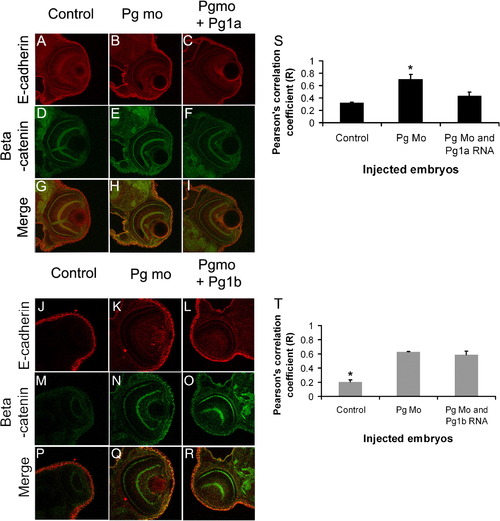
Increased colocalisation of β-catenin and E-cadherin in adherens junctions of plakoglobin morphant embryos. (A–R) Laser-scanning immunofluorescence microscopy images of transverse sections of the eye region of 72 hpf embryos. Dual label fluorescence images with anti-E-cadherin antibody as red and anti-β-catenin antibody as green are shown as (A–F and J–O) single and as (G–I and P–R) merged images. Images are 400x magnification. (G) In control morpholino injected embryos, there was little colocalisation of E-cadherin and β-catenin. (H) By contrast, in plakoglobin morphant embryos there was extensive colocalisation (yellow). (I) This colocalisation was reduced in plakoglobin morpholino and plakoglobin-1a co-injected embryos. (R) However, this colocalisation was still evident in plakoglobin morpholino and plakoglobin-1b co-injected embryos. (S) Quantification of co-localisation of E-cadherin and β-catenin in control, morphant, plakoglobin morpholino and plakoglobin-1a, plakoglobin morpholino and plakoglobin-1b co-injected embryos. There was increased colocalisation of the proteins in morphant embryos compared to control morpholino injected embryos. (S) This co-localisation in co-injected morpholino and plakoglobin-1a RNA injected embryos was similar to control injected embryos. (T) In contrast, levels of co-localisation in plakoglobin morpholino and plakoglobin-1b co-injected embryos were similar to morphant embryos. P < 0.05. Asterisks indicate statistical significance. PHENOTYPE:
|
|
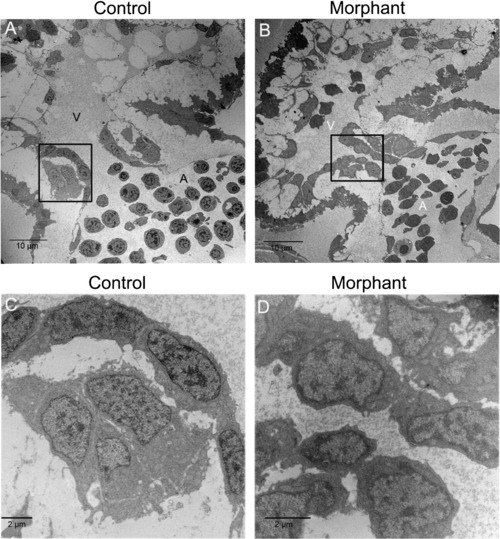
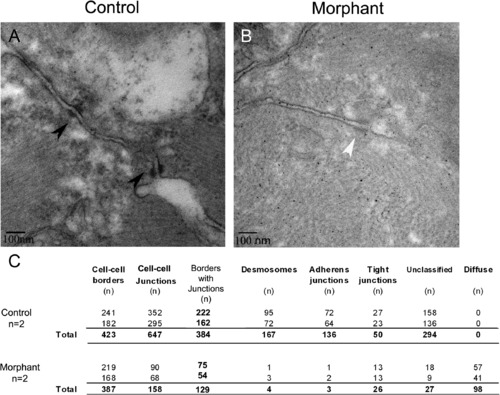
Loss of plakoglobin results in fewer adhesion junctions and with an altered structure. (A and B) Electron microscopy of the intercalated discs of zebrafish embryo hearts at 72 hpf. (A) In control morpholino injected embryos, desmosomes (black arrowheads) were in series. (B) In morphant embryos, diffuse adhesion junctions (white arrowhead) were detected. (C) A table of indicating the cell–cell borders numbers, the number of adhesion junctions, and the number of each type of junction in control and morphant embryos. Unclassified indicates adhesion junctions that could not be definitively classed as adherens junction, desmosome or tight junction. N = 2 embryos per sample. Scale bar is 100 nm. PHENOTYPE:
|
|
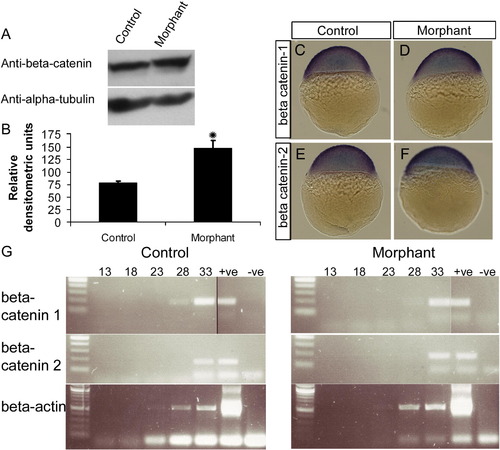
Loss of plakoglobin increases β-catenin protein expression but does not affect β-catenin RNA expression. (A) Western blot analysis of 72 hpf control- or plakoglobin- morpholino injected embryos using a polyclonal anti-β-catenin antibody showing an increase in β-catenin expression in morphant embryos. An anti α-tubulin antibody was used as a loading control. (B) Graph of relative densitometric units of β-catenin expression normalised to α-tubulin levels in control and morphant embryos. (C–F) β-catenin-1 and β-catenin-2 localisation was examined in sphere stage embryos. Expression of β-catenins was similar in morphant embryos and control injected embryo. (G) Semi-quantitative RT-PCR amplification from 24 hpf plakoglobin and control morpholino injected embryos. Lane order: 100 bp ladder, PCR cycle 13, 18, 23, 28, 33, positive plasmid control and negative template control. β-actin was used as a template control. β-catenin-1 or -2 RNA expression in morphant embryos were similar to control-morpholino injected embryos. |
|
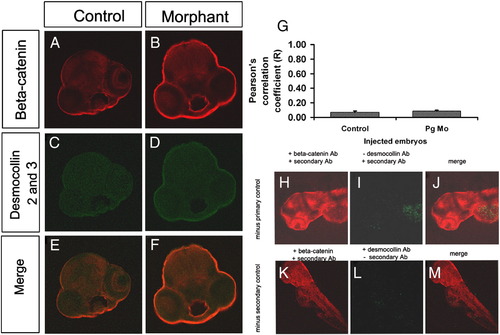
Colocalisation of β-catenin and desmocollin is unaltered in plakoglobin morphant embryos. (A–F) Laser-scanning immunofluorescence microscopy images of transverse sections of the eye region of 72 hpf embryos. Fluorescence images with anti-β-catenin antibody (red), anti-desmocollin 2 and 3 antibody (green) and merged dual images (E and F) are shown. Images are 100x magnification. (A, C, E) In control morpholino injected embryos and (D, E, F) plakoglobin morphant embryos there was little colocalisation of β-catenin and desmocollin 2 and 3. (G) Quantification of co-localisation of E-cadherin and β-catenin in control and morphant embryos. There was a similar level of co-localisation of β-catenin and desmocollin 2 and 3 in control and morphant embryos. Antibody controls for desmocollin 2 and 3 antibody. (H) β-catenin staining in the absence of desmocollin primary antibody. (I) Desmocollin staining in the absence of the desmocollin primary antibody. (J) Merged image of both β-catenin and desmocollin staining. (K) β-catenin expression in the absence of desmocollin secondary antibody control. (L) Desmocollin 2 and 3 staining in the absence of its secondary antibody. (M) Merged image of both β-catenin and desmocollin 2 and 3 staining in the absence of desmocollin secondary antibody. Little staining was detected in either the absence of desmocollin primary or secondary antibody indicating that the primary antibody and the secondary antibody are specific. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 327(1), Martin, E.D., Moriarty, M.A., Byrnes, L., and Grealy, M., Plakoglobin has both structural and signalling roles in zebrafish development, 83-96, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.