- Title
-
Sequential and cooperative action of Fgfs and Shh in the zebrafish retina
- Authors
- Vinothkumar, S., Rastegar, S., Takamiya, M., Ertzer, R., and Strähle, U.
- Source
- Full text @ Dev. Biol.
|
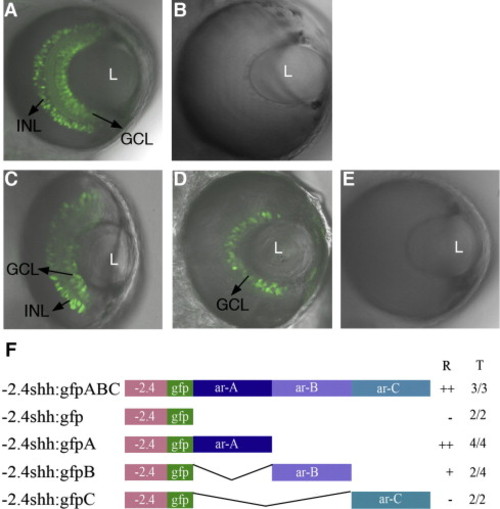
Expression patterns of shh transgenes in the retina. Transgenic lines -2.4shh:gfpABC and -2.4shh:gfpA drive expression in the ganglion cell layer (GCL) and inner nuclear layer (INL) of the zebrafish retina (A, C). In the -2.4shh:gfpB transgenic line, GFP expression is seen only in the GCL (D). The -2.4shh:gfp and -2.4shh:gfpC (B, E) transgenes do not drive GFP expression in the retina. ‘L’ denotes lens. Anterior is to the top in all images. Confocal images taken at 72 hpf. (F) Outline of the enhancer constructs. ‘R’ indicates the expression in the retina and ‘T’ the number of stable transgenic lines showing retina expression out of the total lines analyzed. Scale bar, 50 μm. ‘++’: Expression in both GCL and INL; ‘+’: expression in GCL; ‘-’ no GFP expression in retina. EXPRESSION / LABELING:
|
|
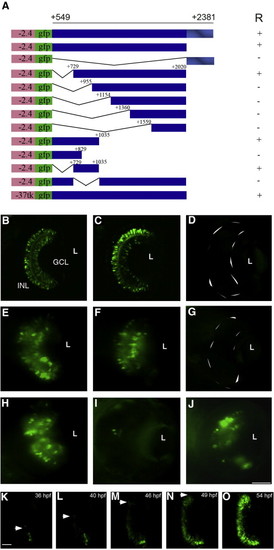
Deletion mapping of the retinal enhancer (RetE). A series of deletion constructs were generated (A) to identify the region required for shh expression in the retina. Transgenes carrying the region from + 549 to + 2381 and + 549 to + 2020 drive expression in the GCL and INL (B, C). The transgene carrying only the conserved region as ar-A from + 2021 to + 2381 failed to show any GFP expression in the retina (D). Transient expression of deletion constructs (E–J). The constructs carrying shh sequence from + 729 to + 2020 and + 549 to + 1035 mediate expression in the retina (E, F). The construct containing shh sequences from + 549 to + 829 did not give retina expression (G). Embryos injected with the -2.4shh:gfpRetE plasmid with the minimal region + 729 to + 1035 have GFP expression in the retina (H), while embryos injected with the -2.4shh:gfp + 549/2020 plasmid with an internal deletion from + 729 to + 1035 failed to drive expression in the retina (I). Embryos injected with the RetE (+ 549 to + 2020) in the context of a heterologous thymidine kinase (-37Tk) promoter -37tk:gfpRetE show retina expression (J). Anterior is to the top in all images and embryos were photographed at 72 hpf. (K–O) In vivo time lapse imaging of -2.4shh:gfpRetE expression in the retina. Single frames taken from a film recording of -2.4shh:gfpRetE expression in the retina, starting at 35 hpf and ending at 54 hpf. The spread of GFP expression is marked by arrowheads. R, retinal expression; L, lens; GCL, ganglion cell layer; INL, inner nuclear layer. Scale bar, 50 μm, panels B–J; 25 μm, panels K–O. +/- Indicates the presence or absence of GFP expression in the retina. |
|
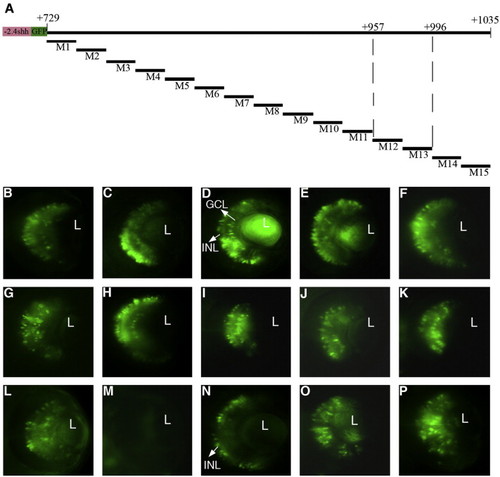
Fine mapping of the 300-bp RetE region. Non-overlapping clusters of point mutations of 20 bp were introduced into -2.4shh:gfpRetE from + 729 to + 1035 of the shh locus (A). The activity of mutant constructs M1–M15 was analyzed by transient expression (B–P). Clustered mutation M12 led to the complete loss of GFP expression (M) whereas mutation M13 abolished GFP expression exclusively in the GCL but not in the INL (N). All other mutant constructs show expression in both layers (B–L, O, P). L, lens; GCL, ganglion cell layer; INL, inner nuclear layer. Scale bar, 50 μm. |
|
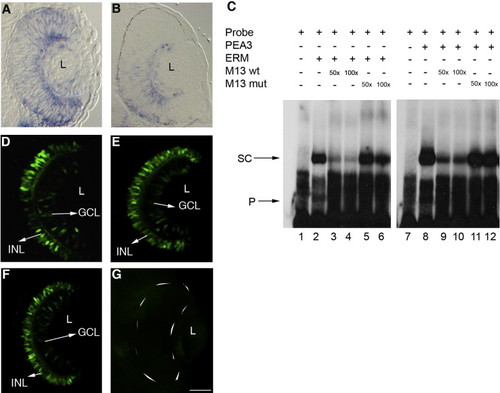
Pea3 and Erm regulate expression in the retina. (A, B) Erm (A) and Pea3 (B) are expressed at low levels throughout the retina with higher levels in the GCL at 34 hpf. (C) In vitro binding of Erm and Pea3 to the RetE probe (lanes 2, 8) and competition using M13 wild-type and M13 mutant oligos (lanes 3–6, 9–12) (SC, shifted complex; P, free probe). (D–G) Transgenic embryos (48 hpf) injected with either MO-pea3 (1.0 μg/μl) or MO-erm (1.0 μg/μl) had no effect on the expression pattern (E, F) while those injected with a mixture of both morpholinos (combined 1.0 μg/μl morpholino) showed a complete loss of GFP expression in the retina (G). Transgenic embryos injected with mismatch morpholinos show normal GFP expression in the GCL and INL (F). Anterior is to the top in all images. L, lens; GCL, ganglion cell layer; INL, inner nuclear layer. Scale bar, 50 μm. EXPRESSION / LABELING:
|
|
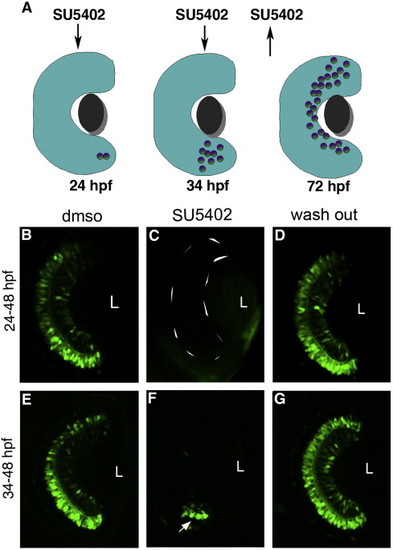
FGF signaling is required for initiation and propagation of GFP expression. (A) Scheme of SU5402 treatment at different time points. (B, E) Treatment of embryos with DMSO at any time does not show alterations in transgene expression. Embryos treated with SU5402 from 24 hpf show a complete loss of expression in the retina (C) while those treated from 34 hpf show a few GFP cells (arrow) at the initiation point (F). Expression recovered when the embryos were removed from SU5402 at 48 hpf and allowed to grow until 72 hpf (D, G). Confocal pictures at 48 hpf (B, C, E, F) and 72 hpf (D, G) with anterior to the top. L, lens. Scale bar, 50 μm. |
|
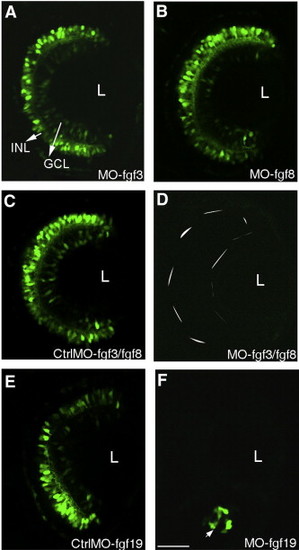
Several Fgfs are required for transgene expression in the retina. Embryos injected with either MO-fgf3 or MO-fgf8 show no effect on the transgene expression in the retina (A, B) while those injected with a mixture of MO-fgf8 and MO-fgf3 block the transgene expression completely (D). Control embryos injected with mismatch MO-fgf3 and mismatch MO-fgf8 have a normal GFP expression pattern (C). Embryos injected with MO-fgf19 show initiation at the ventral nasal patch (arrow) and subsequent spread is blocked (F). Control embryos injected with mismatch MO-fgf19 had a normal GFP expression pattern (E). Confocal pictures at 48 hpf with anterior to the top. L, lens. Scale bar, 50 μm. |
|
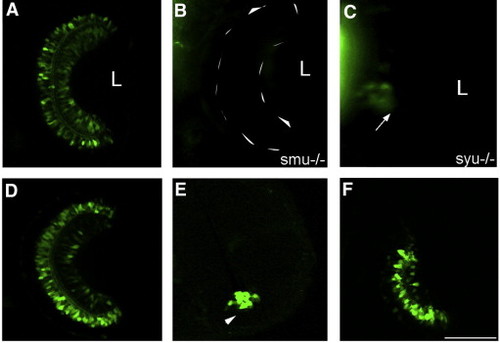
Shh is required for transgene expression in the retina. (A–C) shh signaling mutants slow muscle omitted (smu) shows complete loss of GFP expression retina (B) while sonic-you can express GFP at the initiation site (arrow) (C) when compared to -2.4shh:gfpABC transgenic line at 72 hpf (A). Embryos treated forskolin from 24 to 48 hpf show only initiation of GFP expression (arrowhead) (E) when compared to control embryos treated with DMSO (D). Forskolin- embryos recover expression in the retina after removal of the chemical as seen at 54 hpf (F). Anterior is top in all images. L, lens. Scale bar, 50 μm. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 314(1), Vinothkumar, S., Rastegar, S., Takamiya, M., Ertzer, R., and Strähle, U., Sequential and cooperative action of Fgfs and Shh in the zebrafish retina, 200-214, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.