- Title
-
Migration and differentiation of muscle stem cells are coupled by RhoA signalling during regeneration
- Authors
- Brondolin, M., Herzog, D., Sultan, S., Warburton, F., Vigilante, A., Knight, R.D.
- Source
- Full text @ Open Biol.
|
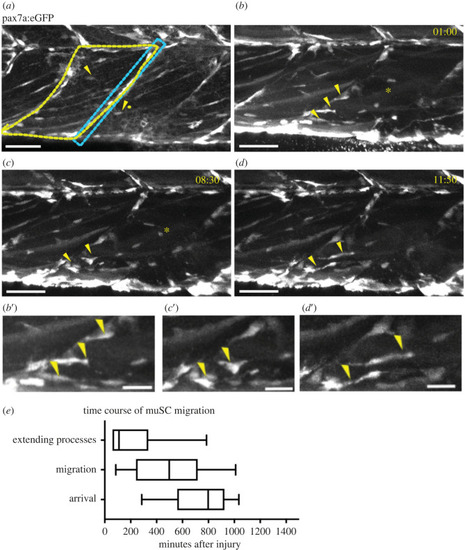
MuSCs show a transition in shape as they move from the myoseptum towards the site of injury (asterisk). GFP + muSCs (arrowheads) in uninjured 7 dpf pax7a:egfp larvae are located at the myoseptum (blue box) or in the myotome (yellow outline, |
|
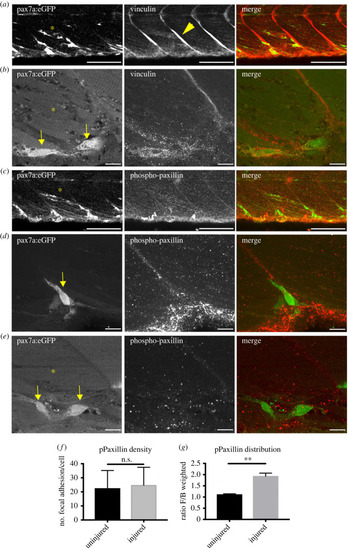
Adhesion molecule localization is polarized in muSCs migrating to injuries. Vinculin is detectable at the myoseptum (arrowheads, |
|
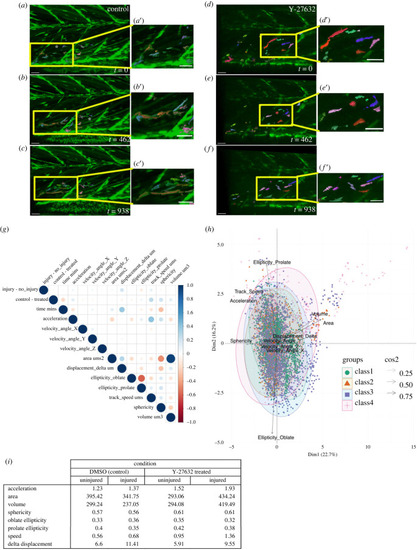
Inhibition of ROCK activity by Y-27632 results in changes to the shape and movement of muSCs responding to injury. Representative images from time-lapsed vidoes of control injured larvae ( |
|
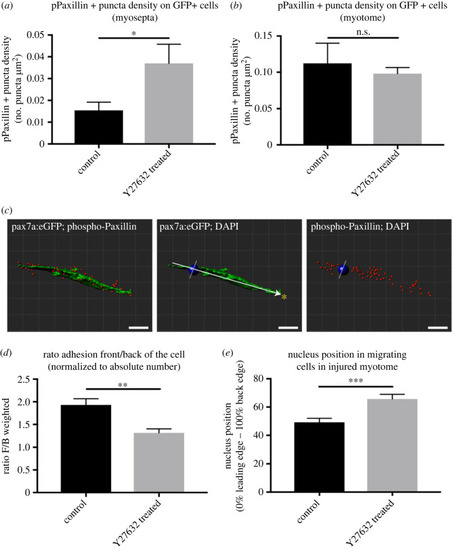
Measures of pPaxillin distribution in GFP+ cells of injured pax7a:egfp larvae in the presence or absence of 50 µM Y-27632. pPaxillin density within GFP+ cells at the myoseptum ( |
|
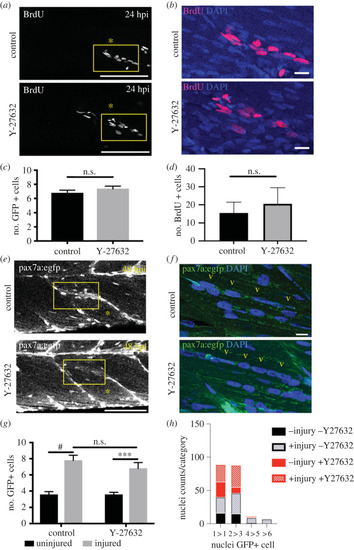
ROCK inhibition does not impair myogenesis during regeneration. Control injured pax7a:egfp larvae (injury location marked by asterisk) or those treated with 10 µM Y-27632 were exposed to BrdU for 24 h ( |
|
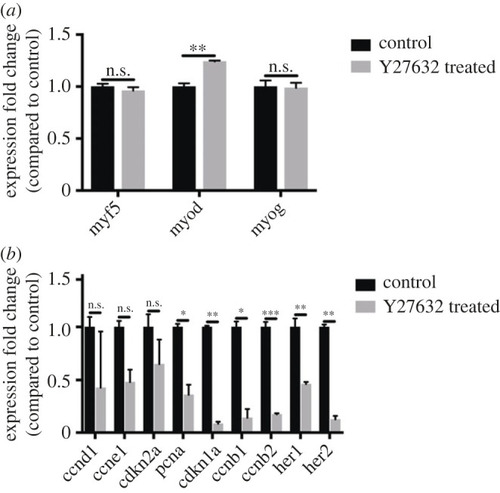
ROCK inhibition impairs myogenesis. Gene expression in regenerating myotomes of control and Y-27632 treated larvae was examined by qPCR at 24 hpi. Myogenic genes |
|
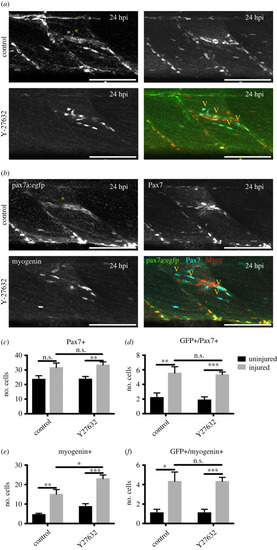
Myogenesis during regeneration of pax7a:egfp larvae was quantified in the absence ( |
|
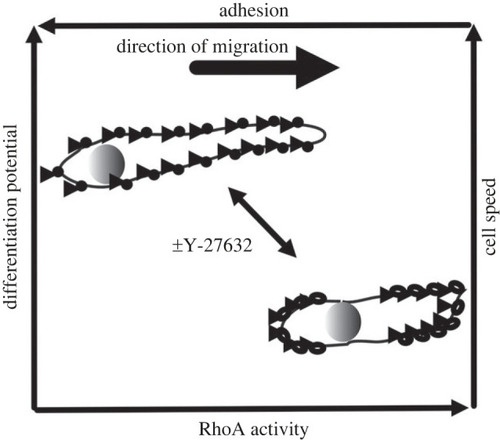
A model for RhoA-dependent migration of muSCs. Migratory muSCs show a RhoA-dependent cell cortex contractility that drives nuclear positioning and cell migration. In an absence of ROCK activity caused by Y-27632, focal adhesions are smaller, less clustered and less polarized along the axis of cell migration leading to reduced adhesion to the extracellular matrix. This correlates with an increased differentiation potential and more rapid migratory behaviour of muSCs. |