- Title
-
Venetoclax is a potent hepsin inhibitor that reduces the metastatic and prothrombotic phenotypes of hepsin-expressing colorectal cancer cells
- Authors
- Rodenas, M.C., Peñas-Martínez, J., Pardo-Sánchez, I., Zaragoza-Huesca, D., Ortega-Sabater, C., Peña-García, J., Espín, S., Ricote, G., Montenegro, S., Ayala-De La Peña, F., Luengo-Gil, G., Nieto, A., García-Molina, F., Vicente, V., Bernardi, F., Lozano, M.L., Mulero, V., Pérez-Sánchez, H., Carmona-Bayonas, A., Martínez-Martínez, I.
- Source
- Full text @ Front Mol Biosci
|
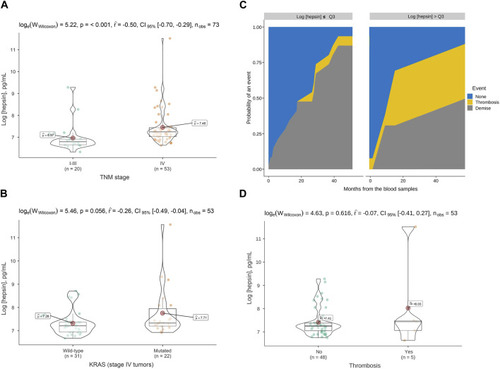
Hepsin levels in plasma of colorectal cancer patients and association with tumor stage, KRAS mutation and thrombosis. |
|
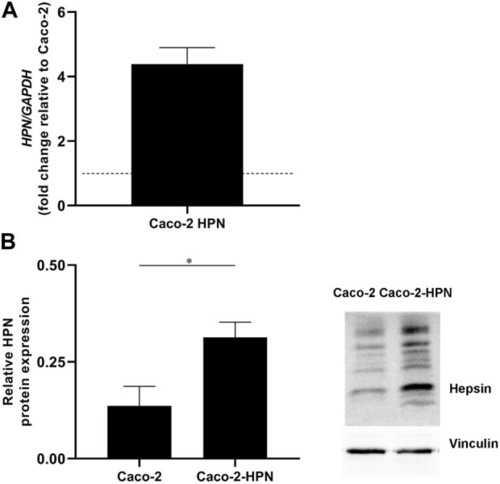
Hepsin overexpression in Caco-2-HPN cells. |
|
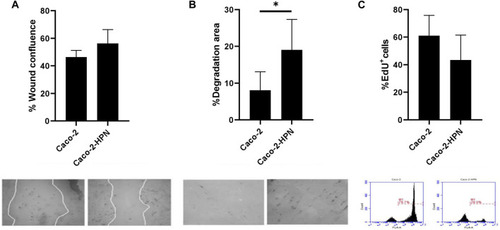
Effects of hepsin levels on cell migration, invasion and proliferation in Caco-2 and Caco-2-HPN cells. |
|
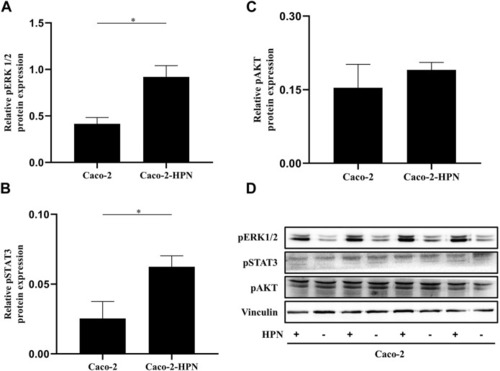
pSTAT3, pAKT and pERK1/2 expression in Caco-2 and Caco-2-HPN cells. Expression of pERK1/2 |
|
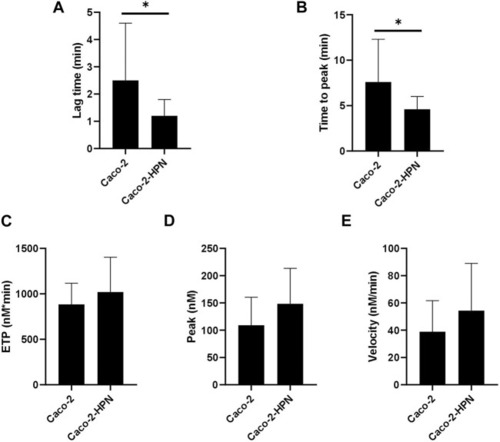
Effects of hepsin levels on thrombin generation by Caco-2 and Caco-2-HPN cells. Thrombin generation was performed after incubation of plasma with cells for 3 h as described in Materials and Methods. Afterwards, plasma was incubated with PPP reagent ® (final concentrations: tissue factor, 1 pmol/L; phospholipids, 4 μmol/L) and calcium chloride. The lag time (min) |
|
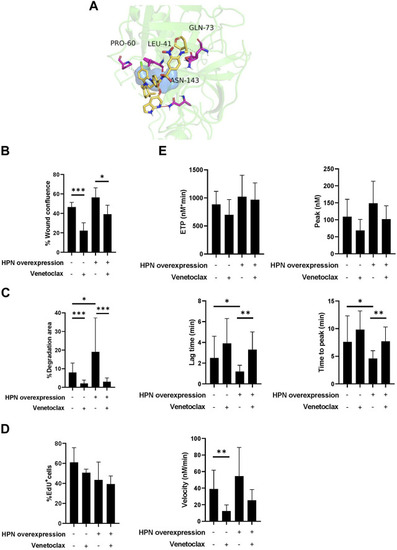
Venetoclax reduces protumor and prothrombotic effects of hepsin in colorectal cancer cells. |
|
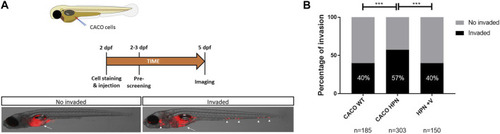
Venetoclax reduces Caco-2-HPN cell invasion in zebrafish larvae. |